Abstract
Purpose
The aim of this study is to evaluate the safety and efficacy of the combination of capecitabine and oxaliplatin (XELOX) as first-line treatment in Chinese patients with metastatic colorectal carcinoma (mCRC). Furthermore, we aimed to explore whether a maintenance therapy with oral capecitabine in patients who were non-progression to the XELOX regimen was able to improve the duration of disease control (DDC).
Patients and methods
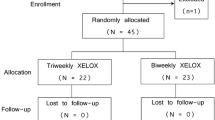
One hundred twenty-four patients with mCRC received a 3-weekly regimen of oxaliplatin plus capecitabine (XELOX) as first-line treatment. Patients without progressive disease after six cycles of XELOX could stop treatment or continue to receive oral capecitabine until disease progression or unacceptable toxicity.
Results
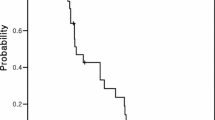
A total of 637 cycles (median 6 cycles) of XELOX were given to 124 patients (males 58.1%, median age 52 years). The response rate was 49.1% (complete response in 11 patients and partial response in 50 patients). The median overall survival and progression-free survival were 20.0 and 8.0 months, respectively. Main drug-related grade 3–4 toxicities included neutrapenia (5.6%), nausea/vomiting (4%), thrombocytopenia (2.4%), diarrhea (2.4%) and hand–foot syndrome (2.4%). Among 62 patients achieving objective response or stable disease after at least 6 cycles of XELOX, there were 22 patients received oral capecitabine as maintenance therapy. The median DDC was significantly longer for maintenance therapy group than those of no maintenance group (14 vs. 9 months; P = 0.041).
Conclusions
XELOX is a highly effective first-line treatment for Chinese mCRC patients. The response rate, TTP, and overall survival of patients treated with this regimen are similar to those treated with FU/leucovorin/oxaliplatin. Furthermore, our preliminary data show maintenance therapy with capecitabine for those patients without progressive disease after at least six cycles of XELOX can significantly improve DDC; and further prospective randomized control trial is warranted.



Similar content being viewed by others
References
Belani CP, Barstis J, Perry MC et al (2003) Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol 21(15):2933–2939
Blum JL, Jones SE, Buzdar AU et al (1999) Multicenter phase II study of capecitabine in paclitaxel refractory metastatic breast cancer. J Clin Oncol 17(2):485–493
Brodowicz T, Krzakowski M, Zwitter M et al (2006) Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: A phase III trial. Lung Cancer 52(2):155–163. doi:10.1016/j.lungcan.2006.01.006
Cassidy J, Twelves C, Van Cutsem E et al (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 13(4):566–575
Cassidy J, Tabernero J, Twelves C et al (2004) XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22(11):2084–2091. doi:10.1200/JCO.2004.11.069
Cassidy J, Clarke S, Díaz-Rubio E et al (2008) Randomized Phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12):2006–2012. doi:10.1200/JCO.2007.14.9898
Caussanel JP, Lévi F, Brienza S et al (1990) Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst 82(12):1046–1050
Chen K, Jing MJ, Jiang QT et al (2006) The molecular epidemiology study of environment exposure and metabolic enzyme gene polymorphism in colorectal cancer. Chin J Epidemiol 27(10):905–908
Comella P, Casaretti R, Sandomenico C et al (2008) Capecitabine, alone and in combination, in the management of patients with colorectal cancer: a review of the evidence. Drugs 68(7):949–961
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Díaz-Rubio E, Tabernero J, Gómez-España A et al (2007) Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol 25(27):4224–4230. doi: 10.1200/JCO.2006.09.8467
Goebel FM, Lledo G, Chibaudel B et al (2007) Final results of OPTIMOX2, a large randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC): A GERCOR study. J Clin Oncol 25(18): abstract 4013
Goh BC, Soo RA, Lim SW et al (2008) Inter-ethnic variability of S-1 pharmacokinetics (PK) and correlation with CYP2A6 phenotyping. J Clin Oncol 26(15S):abstract 2507
Goldberg RM, Sargent DJ, Morton RF et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22(1):23–30. doi:10.1200/JCO.2004.09.046
Jemal A, Murray T, Ward E et al (2005) Cancer statistics, 2005. CA Cancer J Clin 55(1):10–30. doi:10.3322/canjclin.55.1.10
Jordan K, Kellner O, Kegel T et al (2004) Phase II trial of capecitabine/irinotecan and capecitabine/oxaliplatin in advanced gastrointestinal cancers. Clin Colorectal Cancer 4(1):46–50. doi:10.3816/CCC.2004.n.009
Mackean M, Planting A, Twelves C et al (1998) Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol 16(9):2977–2985
Makatsoris T, Kalofonos HP, Aravantinos G et al (2005) A phase II study of capecitabine plus oxaliplatin (XELOX): a new first-line option in metastatic colorectal cancer. Int J Gastrointest Cancer 35(2):103–109
Miwa M, Ura M, Nishida M et al (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34(8):1274–1281
Nakajima M, Fukami T, Yamanaka H et al (2006) Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther 80(3):282–297. doi:10.1016/j.clpt.2006.05.012
Novello S, Bruzzi P, Barone C et al (2007) Phase III study in stage IV non-small cell lung cancer patients treated with two courses of cisplatin/gemcitabine followed by a randomization to three additional courses of the same combination or gemcitabine alone. Ann Oncol 18(5):903–908. doi:10.1093/annonc/mdm061
Scalamogna R, Brugnatelli S, Tinelli C et al (2007) UFT as maintenance therapy in patients with advanced colorectal cancer responsive to the FOLFOX4 regimen. Oncology 72(5–6):267–273. doi:10.1159/000113037
Scheithauer W, Kornek GV, Raderer M et al (2003a) Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 21(7):1307–1312
Scheithauer W, McKendrick J, Begbie S et al (2003b) Oral capecitabine as an alternative to i.v. 5-fluorouracilbased adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol 14(12):1735–1743
Twelves C, Wong A, Nowacki MP et al (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352(26):2696–2704
Van Cutsem E, Hoff PM, Harper P et al (2004) Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: Integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer 90(6):1190–1197. doi:10.1038/sj.bjc.6601676
Acknowledgments
The authors would like to thank the staff members in the Department of Medical Oncology and Department of Abdominal Surgery at Sun Yat-Sen University Cancer Center for their valuable suggestion and assistance.
Grant support: Major science and technology project of “National Significant new drug creation”, No. 2008ZX09312-002, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y.H. Li and H.Y. Luo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Y.H., Luo, H.Y., Wang, F.H. et al. Phase II study of capecitabine plus oxaliplatin (XELOX) as first-line treatment and followed by maintenance of capecitabine in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol 136, 503–510 (2010). https://doi.org/10.1007/s00432-009-0682-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0682-5