Abstract
Background
The aim of this multicenter, open-label, randomized phase II trial was to evaluate the efficacy of a dose-dense capecitabine and oxaliplatin (XELOX) regimen in patients with metastatic colorectal cancer (mCRC) for whom reintroduction of oxaliplatin had been planned as a third- or later-line regimen.
Methods
The patients with mCRC who had received prior chemotherapy including oxaliplatin and were scheduled for reintroduction of oxaliplatin were randomized to capecitabine (1,000 mg/m2) twice daily on days 1–14 and oxaliplatin (130 mg/m2) on day 1 every 21 days (Q3W group) or capecitabine (2,000 mg/m2) twice daily on days 1–7 and oxaliplatin (85 mg/m2) on day 1 every 14 days (Q2W group). The primary endpoint was the time-to-treatment failure (TTF). Other endpoints included overall survival (OS), progression-free survival (PFS) and other adverse events (AEs).
Results
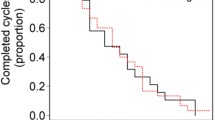
A total of 46 patients were enrolled in the trial—22 patients were randomly assigned to the Q3W group and 23 to the Q2W group. The median TTF was 3.4 months in both groups (hazard ratio [HR] 1.053; p = 0.880). The median PFS and OS were 3.3 and 9.2 months in the Q2W group and 4.3 and 12.1 months in the Q3W group, respectively (HR 1.15; p = 0.153 and 0.672; p = 0.836). The most common grade 3−4 AEs in the Q3W and Q2W groups were fatigue (27.3 vs 21.7), neuropathy (9.1 vs 0 %) and diarrhea (9.1 vs 0 %), respectively.
Conclusion
There was no significant inter-group difference in any of the efficacy and safety endpoints, including TTF, OS, RFS and AEs. The results of this clinical trial were convincingly negative.


Similar content being viewed by others
References
Goldberg RM, Sargent DJ, Morton RF et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Benson AB 3rd, Venook AP, Bekaii-Saab T et al (2014) Colon cancer, version 3.2014. J Natl Compr Canc Netw 12:1028–1059
de Gramont A, Buyse M, Abrahantes JC et al (2007) Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol 25:3224–3229
Tournigand C, Cervantes A, Figer A et al (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer a GERCOR study. J Clin Oncol 24:394–400
Cassidy J, Clarke S, Diaz-Rubio E et al (2011) XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer 105:58–64
Zhang C, Wang J, Gu H et al (2012) Capecitabine plus oxaliplatin compared with 5-fluorouracil plus oxaliplatin in metastatic colorectal cancer: meta-analysis of randomized controlled trials. Oncol Lett 3:831–838
Scheithauer W, Kornek GV, Raderer M et al (2002) Intermittent weekly high-dose capecitabine in combination with oxaliplatin: a phase I/II study in first-line treatment of patients with advanced colorectal cancer. Ann Oncol 13:1583–1589
Scheithauer W, Kornek GV, Raderer M et al (2003) Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 21:1307–1312
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Hurwitz H, Mitchell EP, Cartwright T et al (2012) A randomized, phase II trial of standard triweekly compared with dose-dense biweekly capecitabine plus oxaliplatin plus bevacizumab as first-line treatment for metastatic colorectal cancer: XELOX-A-DVS (dense versus standard). Oncologist 17:937–946
Grande C, Quintero G, Candamio S et al (2013) Biweekly XELOX (capecitabine and oxaliplatin) as first-line treatment in elderly patients with metastatic colorectal cancer. J Geriatr Oncol 4:114–121
Kakolyris S, Souglakos J, Polyzos A et al (2008) Modified CAPOX (capecitabine plus oxaliplatin) regimen every two weeks as second-line treatment in patients with advanced colorectal cancer previously treated with irinotecan-based frontline therapy: a multicenter phase II study. Oncology 74:31–36
Grothey A (2010) Reintroduction of oxaliplatin: a viable approach to the long-term management of metastatic colorectal cancer. Oncology 79:389–399
Loprinzi CL, Qin R, Dakhil SR et al (2014) Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 32:997–1005
Kono T, Hata T, Morita S et al (2013) Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother Pharmacol 72:1283–1290
Acknowledgments
This work was supported, in part, by a non-profit organization, Epidemiological & Clinical Research Information Network (ECRIN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
C. Matsuda and M. Honda contributed equally to this study.
About this article
Cite this article
Matsuda, C., Honda, M., Tanaka, C. et al. Multicenter randomized phase II clinical trial of oxaliplatin reintroduction as a third- or later-line therapy for metastatic colorectal cancer—biweekly versus standard triweekly XELOX (The ORION Study). Int J Clin Oncol 21, 566–572 (2016). https://doi.org/10.1007/s10147-015-0911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0911-7