Abstract
Congenital portosystemic venous shunts are rare developmental anomalies resulting in diversion of portal flow to the systemic circulation and have been divided into extra- and intrahepatic shunts. They occur during liver and systemic venous vascular embryogenesis and are associated with other congenital abnormalities. They carry a higher risk of benign and malignant liver tumors and, if left untreated, can result in significant medical complications including systemic encephalopathy and pulmonary hypertension.
Conclusion: This article reviews the various types of congenital portosystemic shunts and their anatomy, pathogenesis, symptomatology, and timing and options of treatment.
What is Known: • The natural history and basic management of this rare congenital anomaly are presented. |
What is New: • This paper is a comprehensive review; highlights important topics in pathogenesis, clinical symptomatology, and treatment options; and proposes an algorithm in the management of congenital portosystemic shunt disease in order to provide a clear idea to a pediatrician. An effort has been made to emphasize the indications for treatment in the children population and link to the adult group by discussing the consequences of lack of treatment or delayed diagnosis. |
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Congenital portosystemic venous shunts (CPSS) are rare vascular anomalies that occur secondary to abnormal development or involution of fetal vasculature. They allow intestinal blood to reach the systemic circulation bypassing the liver, resulting in a variety of symptoms and complications in the longer term [1]. They usually occur as isolated malformations, but multiple shunts may exist. Ascites and portal hypertension are not usually features of CPSS, in contrast to secondary portosystemic shunts in the setting of liver cirrhosis or portal vein occlusion [2]. CPSS are divided into intra- and extrahepatic shunts. Although their clinical manifestations may be similar, the pathophysiology and treatment of the two types differ.
The overall incidence of CPSS is estimated to be 1:30,000 births and 1:50,000 for those that persist beyond early life [3]. The prevalence of intrahepatic shunts is estimated to be 0.0235% as reported from a random ultrasonography screening population sample of asymptomatic adults [4]. In a study of 145,000 newborns in Switzerland, 5 cases of CPSS were identified [5].
Abernethy described a case of an extrahepatic shunt in the postmortem of a 10-month-old female patient in 1793. The portal vein was noted to terminate in the inferior vena cava (IVC) at the level of renal veins. Several other abnormalities were also found in association with the shunt [6].
Embryology
The primitive liver emerges within an epithelial and mesenchymal interactive network of three basic embryological venous systems (cardinal, vitelline, and umbilical veins). Anterior and posterior cardinal veins and the two vitelline veins comprise the origin of the future systemic and portal venous systems, respectively, whereas the umbilical veins drain the yolk sac and placenta before their final regression [3, 7].
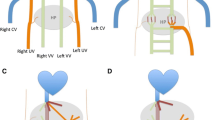
The development of the portal system is complex and occurs between the 4th and 10th week of embryonic life. Initially, the two vitelline veins emerge from the anterior surface of the yolk sac and drain to the sinus venosus. By the end of the 4th week, they create at least three cross-communicating channels (subhepatic-cranioventral duodenal, intermediate-dorsal duodenal, and caudal-ventral duodenal) around the developing duodenum in order to form the vitelline venous network. In the septum transversum, cords surround the intrahepatic component of the newly developing vitelline system in order to give rise to hepatic sinusoids, which selectively involute to form the final configuration of intrahepatic branches of portal and hepatic veins at 8th week [7,8,9]. Between the 10th and 12th week, the left vitelline vein disappears, and the cranial part of the right vitelline vein and the segment that lies inferior to the liver give rise to the terminal branch of the IVC and portal and superior mesenteric veins, respectively [7,8,9,10] (Fig. 1).
Diagram showing development of the portal and hepatic veins. The two vitelline veins communicate inside the liver and around the duodenum to form intrahepatic portal and hepatic veins: the left vitelline vein disappears, and the cranial part of the right vitelline vein and the segment that lies inferior to the liver give rise to the terminal branch of the IVC and portal and superior mesenteric veins. Incomplete involution and persistent communication of the vitelline venous system during the development of newly formed hepatic sinusoids results in various types of portosystemic shunts [11]
Incomplete involution of the vitelline venous system in response to the development of hepatic sinusoids is probably the main reason for shunt formation and depends on the anatomical site (right or left) and level (proximal or distal) at which the vitelline veins fail to differentiate. Intrahepatic shunt type 1 and extrahepatic type 2 side to side variants arise from persistence of the right vitelline vein, whereas other extrahepatic shunts draining into the IVC above the level of the hepatic vein confluence, or to the right atrium appear to be due to persistence of the left vitelline vein. Other authors have suggested that failure of remodeling of the anastomotic channels between vitelline and subcardinal veins during the development of the IVC in the early embryonic stages may play a role in creating type 2 extrahepatic shunts [8, 12, 13].
Extrahepatic shunt type 1 (Abernethy malformation) with the “absence” of the intrahepatic portal veins is thought to be the result of excessive involution of the periduodenal vitelline plexus. Similarly, persistent communication of the vitelline venules within the newly formed hepatic sinusoids results in type 2–4 intrahepatic shunts [8].
The ductus venosus connects the umbilical vein and the inferior vena cava during embryonic life. It arises from the posterior aspect of the left portal vein PV, opposite the opening of the umbilical vein and drains into the left hepatic vein near its entry into the IVC (or directly to the IVC) [14]. Spontaneous closure begins immediately after birth and is completed during the first week of life. Delayed closure may occur due to an alteration in hemodynamics from congenital heart defects, and a patent ductus venosus acts as an intrahepatic shunt and may result in hypoplasia of the portal vein [15, 16].
Other abnormalities associated with CPSS
CPSS are associated with multiple congenital abnormalities, with the most common involving the cardiovascular system, and include ventricular and atrial septal defects (VSD, ASD), patent foramen ovale, coarctation of the aorta, tetralogy of Fallot, and patent ductus arteriosus (PDA). [16]. They potentially affect liver hemodynamics and contribute to the creation and maintenance of these portosystemic shunts (e.g., cardiac defects and patent ductus venosus) [16].
Another common abnormality is the polysplenia syndrome with azygos or hemiazygos continuation of the inferior vena cava, which is found in 8% of extrahepatic shunt cases. Screening for CPSS should be performed in all patients with polysplenia [3, 17]. Other vascular abnormalities associated with CPSS include splenic artery aneurysms, coronary artery fistulas, primitive hypoglossal artery, and cutaneous hemangiomas [18].
Other abnormalities and syndromes, which have been associated with CPSS, are summarized in Table 1. The majority have been associated with extrahepatic shunts, whereas anomalies with intrahepatic shunts are less frequent and limited to cardiac, renal, and biliary anomalies, vascular aneurysms, and a small number of rare syndromes (e.g., Trisomia 21, Leopard, and Rendu-Osler-Weber) [3, 7, 19, 21].
Classification
Classification of CPSS can be challenging as significant variety and complexity, in terms of localization, configuration, size, vessels involved, number of pathways, and the presence of intrahepatic portal branches, exists. CPSS were historically classified by their anatomy; however, continued improvements in our understanding of the pathophysiology of the condition are helping to guide management. Extrahepatic shunts have also been classified according to criteria based on their clinical presentation and liver histopathology, which guide subsequent treatment [22].
For intrahepatic portosystemic shunts, the classification described by Park et al. appears to be the most descriptive and defines them as communications > 1 mm in diameter between the intrahepatic portal vein and the hepatic or perihepatic veins. Four types have been identified: a single vessel communication, which can be either between a main branch of the portal vein and IVC (type 1), peripheral location in one segment (type 2), or through an aneurysm (type 3), and multiple small communications distributed diffusely in both lobes (type 4) [23]. They appear to have a male predominance, with the first two varieties being the most common. A patent ductus venosus is invariably referred to as an intrahepatic shunt type 5, despite its course in the ligamentum venosum, because it originates from the left portal vein [11].
In the classification of extrahepatic portosystemic shunts, no difference has been reported in the incidence between male and female patients [20]. Based on evidence of portal flow to the liver, extrahepatic portosystemic shunts (EPSS) have been classified into type 1 “Abernethy malformations” with an end to side shunt and an apparent absence of any portal branches to the liver, and type 2 in which portal flow is partially diverted to systemic circulation with either a preserved or hypoplastic main portal trunk connecting to the IVC in a side to side fashion [24]. Type 1 can be further subdivided based on whether the superior mesenteric and splenic vein drain separately (type 1a) or via a common trunk (type 1b) to IVC or less commonly to another systemic vein (e.g., azygos, iliac, renal) [25].
A critical factor in the classification of CPSS is the presence of portal flow to the liver which can be determined by the evidence of patency of extrahepatic and intrahepatic portal branches. Assessment depends on cross-sectional imaging, the use of an occlusion test, and results from liver biopsy to understand the degree of hypoplasia of the intrahepatic portal venous system [26,27,28]. Complete extrahepatic or intrahepatic shunts, which with conventional imaging appear to show an absent portal flow to the liver, may reveal a previously hypoplastic intrahepatic portal system which gradually opens up after venogram and shunt occlusion. This has been proposed as an essential aspect of early assessment and management of all cases [26,27,28].
Other authors have proposed additional classification patterns in their effort to understand the pathophysiology of the condition and to help guide management. Kanazawa et al. proposed an alternative classification, based on the severity of the intrahepatic portal hypoplasia (mild, moderate, and severe) and the portal pressure at shunt occlusion, which would reflect the clinicopathological features and provide useful information about likely response to therapy (Table 2) [29]. Other classifications include those described by Lautz et al. based on the origin of the shunt in the portal circulation (Table 3), and Kobayashi et al. whose classification was based on the systemic site of drainage in relation to symptoms etiology (gastrointestinal bleeding, encephalopathy, liver tumors) (only extrahepatic Table 4) and that of Blanc et al. which focused on the shunt caval ending and the indications for surgical closure in either one or two stages based on the communication pattern (end to side vs side to side) (Table 5) [19, 30, 31]. A more detailed and accurate but possibly less practical anatomical description of both intra- and extrahepatic shunts was reported by Bernard et al. who reviewed 265 pediatric cases and classified them according to the site of shunt origin (portal vein, afferent and efferent branches), the ending systemic vein drainage, and the pattern and number of communications [3].
Clinical picture
Patients with CPSS present with a wide spectrum of symptoms and complications that may occur during life, although asymptomatic cases, discovered incidentally on imaging, are not uncommon. Hepatic encephalopathy, hepatopulmonary syndrome, and pulmonary hypertension are the most prominent manifestations caused by long-term portosystemic shunting and are more often observed in children [20, 32].
Even before birth, alterations in fetal venous circulation from shunting may result in decreased liver perfusion and signs of intrauterine growth restriction in the absence of hypoxia or other obvious maternal infections and/or chromosomal abnormalities [33]. Neonatal cholestasis and galactosemia may occur and should be differentiated from other congenital defects such as biliary atresia and metabolic disorders which may also coexist [34, 35].
Children with CPSS may present with unexplained neurocognitive dysfunction and other behavioral issues due to low-grade hepatic encephalopathy and this accounts for between 17 and 30% of cases [8]. Other manifestations include learning disabilities, extreme fatigability, seizures, and failure to thrive and have been associated with elevated arterial ammonia levels in the majority of cases. The likelihood of encephalopathy increases with age and is related to the shunt flow [8, 14].
Refractory hypoxia and hepatopulmonary syndrome can be found in about 10% of cases. Potent vasoactive mediators when bypassing the liver cause intrapulmonary vascular dilatation and impaired oxygen exchange. Patients usually present with cyanosis, digital clubbing, and dyspnea on exertion or at rest [36]. Associated portopulmonary hypertension can affect 13–66% of children with hepatopulmonary syndrome and CPSS and usually appears later in the course of the disease [7, 37, 38]. The histological picture is consistent with obliteration of pulmonary arteries with microthrombi and intimal fibrosis. The degree of hypertension does not seem to correlate with the shunt size and may possibly be secondary to a coexisting cardiac anomaly. Portopulmonary hypertension carries a poor prognosis with a reported mortality of 50% due to late identification and failure to reverse even after shunt closure [7, 37, 38].
Regenerating liver nodules (e.g., adenoma, focal nodular hyperplasia, hemangioma) have been reported as a result of the alteration in local hemodynamics, with the compensatory increase in arterial flow, and associated elevated circulating levels of hepatic growth factors (e.g., insulin, glucagon, hepatocyte growth factor) are seen in 25–50% of CPSS cases [39, 40]. They can be single or multiple, can occur at any age, and are most commonly found in adult patients with EPSS. Malignant tumors (hepatocellular carcinoma (HCC), hepatoblastoma, sarcoma) have been reported to occur in these patients in the absence of liver dysfunction and cirrhosis (4% of all cases) [41]. They appear exclusively with extrahepatic shunts and may occur as de novo primary tumors, but transformation of preexisting benign lesions seems to be more common. Hepatoblastomas are rare tumors and are associated with other genetic disorders as well. Cases in which they occur after transformation of preexisting focal nodular hyperplasia have been reported [41]. The risk of primary HCC in patients with extrahepatic shunts seems to be similar to that of liver cirrhosis. In most cases, the alpha-fetoprotein is elevated and the shunt appears to work as an independent risk factor in the absence of chronic liver disease [42, 43]. In addition, benign tumors such as adenoma or focal nodular hyperplasia show a different pathogenesis and natural history than conventional tumors and seem to carry a higher risk of malignant transformation. This is supported by the evidence of specific mutations (beta-catenin mutations resulting in activation of various transcription factors) discovered in hepatocytes possibly in association with the altered hemodynamics. For these reasons, indications for treatment differ and close surveillance of those nodules is recommended [44].
Gastrointestinal bleeding as a presenting symptom has been reported in 8.1% of cases with EPSS. In the majority of these cases, the ending systemic veins of the shunt were the iliac veins, resulting in colonic and rectal varices [45]. Encephalopathy and liver tumors were uncommon in this group of patients perhaps suggesting that the shunt was only partial and provided a degree of protection.
Children that were undiagnosed or put under long-term surveillance due to mild symptomatology and remained without any treatments may develop clinical symptoms at a later age with the likelihood to increase over 50 years. Adults usually show mild to moderate neurological impairment with features similar to patients with chronic liver disease. Other symptoms such as abdominal pain and hypoglycemia may be seen, too. Unusual findings such as parkinsonism, autism, and spastic paraparesis have also been described in association with hyperammonemia [46,47,48]. Asymptomatic patients with a low shunt function may develop symptoms of encephalopathy when precipitating factors such as gastrointestinal bleeding or constipation elevate blood ammonia levels [46,47,48].
Complications that have been reported with CPSS include hyperandrogenism caused by hyperinsulinemia due to insulin resistance from the altered liver hemodynamics (in a similar manner to liver cirrhosis), pancreatitis (associated anatomical narrowing of the pancreatobiliary junction), vaginal bleeding, and lower urinary tract symptoms (lithiasis, hematuria). A rare complication that has been described is membranoproliferative glomerulonephritis (MPGN), a well-known manifestation that usually can be seen in cirrhotic patients postdecompression of the portal system (e.g., surgical portocaval shunt) and subsequent reduced hepatic clearance of immune complexes [37, 49, 50].
Diagnosis
A comprehensive workup comprising radiological, biochemical, and dynamic invasive tests are invariably required to establish the diagnosis and delineate the shunt anatomy. The selection of each test depends on the age of the children, the anatomical complexity of the shunt, the clinical signs and complications, and the potential for treatment at the same time. The initial tests should include Doppler ultrasound and arterial ammonia levels. Enhanced color Doppler ultrasound will also allow for estimation of the shunt ratio by dividing the blood flow volume at the shunt orifice by the total portal flow [51]. In the past, rectal scintigraphy was used to calculate the ratio uptake of rectally delivered isotope (iodine 123 iodoamphetamine) between the lungs and the liver as an indirect measurement of the amount of blood that passes through the shunt. Shunt ratios of greater than 5% were considered abnormal [13, 15]. This method has gradually been replaced by the Doppler ultrasound although one study demonstrated (Yuki Cho et al.) superiority in the estimation of shunt severity using rectal scintigraphy [12, 14, 52].
The level of serum ammonia varies, and although it is proportional to the degree of shunt flow and normalizes after shunt occlusion, studies have shown that it does not always correlate with the degree of encephalopathy [7, 8]. Moreover, it has been reported to be normal in some patients even in the presence of overt neurological symptoms. Repeated measurement of ammonia levels is required.
Additional tests may support further the diagnosis and help in assessing the severity of symptoms (e.g., encephalopathy). Newborn children may be diagnosed with a CPSS on routine screening for galactosemia without enzyme deficiency [34]. An oral glutamine challenge test may precipitate encephalopathy and define the problem more clearly. Magnetic resonance imaging of the brain may reveal white matter atrophy and abnormal signals on the T1-weighted images of the basal ganglia, characteristically at the globus pallidus, which correspond to manganese deposition. This finding has been reported in the absence of clinical encephalopathy in a patient with CPSS. Electroencephalography and other neuropsychological tests are invaluable, as low-grade encephalopathy is often misdiagnosed as behavioral problems [8, 21, 53].
Abdominal imaging (computed tomography or magnetic resonance imaging) is used initially to delineate shunt anatomy and characterize potential focal liver lesions. This may indicate the absence of intrahepatic portal network; therefore, in such cases, invasive tests (e.g., mesenteric portovenography) are required to evaluate the intrahepatic portal system plasticity via an occlusion test. Definitive occlusion of the shunt at the same time, depending on portal hemodynamics, is an option for favorable cases. If this is not feasible, data from the occlusion test can be utilized for planning future intervention and final treatment modality [7, 54].
Liver biopsy may show atrophy of the liver, due to portal flow deprivation and lack of hepatotrophic factors; however, findings of advanced fibrosis or cirrhosis are rarely if ever seen. Complete or near complete absence of the portal venules and hypertrophy of hepatic artery branches and bile duct proliferation, with or without nodular regenerative hyperplasia, are common finding especially with type 1 extrahepatic shunts. These findings help in predicting the potential for expansion of portal venules after shunt occlusion [54]. According to Kanazawa et al., the severity of intrahepatic hypoplasia of the portal network correlates with the size and not the number of portal triads as it was found that the number was similar in the mild, moderate, and severe types of hypoplasia. Small-sized portal triads were seen extensively in the severe type as opposed to the moderate (occasionally) and mild types (rarely) in which portal venules were constricted and distorted in a crescent shape. In this study, 13 patients with mild or moderate hypoplasia and 2 with severe hypoplasia tolerated definitive radiological occlusion of an extrahepatic shunt in a single procedure [29]. Liver biopsy in the setting of an indeterminate or increasing size liver nodule may also be necessary. Serum alpha-fetoprotein levels are mandatory in helping to evaluate focal liver lesions or screen at-risk patients in combination with ultrasonography. In addition, other tests may be useful to assess potential underlying liver disease especially in adult patients with spontaneous shunts (elastography, hepatitis screen, etc) [41, 54,55,56,57].
Further tests to identify and assess potential complications in other organs or to investigate associated congenital anomalies (e.g., cardiac echo, GI endoscopy, lung studies) are routinely used.
Treatment
CPSS are rare abnormalities and large series which have adopted a standard therapeutic approach are not currently available. The shunt type, location and degree of function, patient age, and the severity of symptoms and complications determine treatment strategy. Conservative management has been proposed in cases with mild symptoms or when spontaneous closure was anticipated. The basic principle of intervention is to disrupt the abnormal communication between portal and systematic circulation and restore portal flow to the liver. The view has emerged that all shunts that persist after the first year of life should be closed without waiting for complications to develop.
Observation and monitoring of arterial ammonia levels may be sufficient in asymptomatic adult patients with low flow shunts who can be followed up with serial Doppler ultrasound [58]. The likelihood of symptoms is proportional to the shunt size and flow (> 30%). Medical management is similar to that used for cirrhotic patients with hepatic encephalopathy including protein restriction, lactulose, and nonabsorbable antibiotics. Previously, it was recommended that monitoring of the shunt size and ammonia level was sufficient for mild symptomatology. However, this has changed and these shunts should also be closed after excluding the presence of significant pulmonary hypertension [58].
Intrahepatic shunts that are diagnosed prenatally or during early infancy do not necessarily require definitive treatment, as many will spontaneously close by the age of 1 year with resolution of symptoms, in contrast to extrahepatic shunts and patent ductus venosus where closure is unlikely [59]. Spontaneous closure of intrahepatic shunts is more often seen in girls, in the presence of multiple shunts and in children with neonatal cholestasis [59].
For children with EPSS or a persistent intrahepatic portosystemic shunt, the optimal timing of treatment has not been defined. Many authors have proposed that even in the absence of overt symptoms, early intervention prevents pulmonary complications and neurodevelopmental delay and intellectual and psychosocial function may be preserved [22]. The presence of clinical encephalopathy, hepatopulmonary syndrome, portopulmonary hypertension, regenerative liver nodules, and evidence of increasing shunt size are clear indications for intervention if the patient is fit enough to tolerate any planned procedure. Cases with refractory symptoms, previous failed medical treatment, or an increased shunt ratio > 60% are likely to benefit from shunt occlusion. The choice of radiological or surgical approach depends on local expertise, shunt anatomy and size, and the patient’s fitness [22, 29].
Interventional radiology is the least invasive method and results in rapid amelioration of symptoms, correction of high ammonia levels, and regression of liver lesions [32, 60, 61]. Catheter insertion via a transhepatic route offers better exposure for a intrahepatic shunt associated with the contralateral portal vein to the initial punctured entry side, when a transcaval route, using either common femoral or internal jugular vein access, is preferred for large intrahepatic shunts close to the inferior vena cava or any type of extrahepatic shunts. A small number of cases using a transileocolic approach via a minilaparotomy have also been described [9].
Embolization of the shunt using various materials including coils and microcoils, detachable balloons, and N-butyl cyanoacrylate lipiodol mixtures or recently introduced multilayer devices (vascular plugs) has been reported depending on shunt size [32, 62, 63]. For small caliber shunts, coils are effective and the risk of migration is low. For larger, high-velocity flow shunts, a vascular plug (e.g., Amplatzer) with a diameter 30 to 50% larger than the fistula size has been used and can be positioned accurately with less metallic artifact on follow-up imaging. This allows better visualization of any persistent venovenous shunting. The combination of more than one material has also been reported as being effective in recurrent or complex shunts when a vascular plug positioned first can be used as an anchor for coils that target small vessels with residual flow [63].
During the procedure, an occlusion test with a temporary balloon placement is recommended to record portal vein pressure elevation. If previous liver biopsy has demonstrated severe hypoplasia of intrahepatic portal system, then portal pressure is expected to be high [38, 64,65,66]. If the portal pressure exceeds 30 mmHg, then permanent occlusion at this stage should be avoided, as it is likely to cause significant changes in liver hemodynamics, derangement of liver function, and potential worsening of preexisting symptoms and gastrointestinal bleeding. A two-stage approach is advocated for these cases with the use of a size reduction stent followed by definitive occlusion (radiological or surgical ligation) a few months later and will allow the liver to compensate and the portal pressure to reduce gradually without complication [38, 64,65,66].
Surgical ligation is an acceptable method and can be used in cases with large shunts with a risk of inadvertent migration of embolic agents during embolization or after failed embolization with shunt recurrence or persistence. Moreover, if the shunt is not long enough as in a side to side communication, then placement of coils or plugs may be difficult [15, 29, 67, 68]. Laparoscopic ligation has also been reported in more peripheral extrahepatic shunts (e.g., splenorenal) [69]. Surgical ligation has some relative limitations in terms of failure to identify intrahepatic or multiple shunts and the risk of acute portal hypertension/thrombosis and subsequent bowel congestion. Blanc et al. have proposed a classification for CPSS based on the largest series of surgical ligations in one or two stages. The authors emphasized that ligation should be as close as possible to the caval system as inadvertently proximal severe portal hypertension and thrombosis of blind portal segments may occur during occlusion. If portal pressure is high (cutoff point 25 mmHg), then temporary banding and completion of ligation in two stages is preferable. End to side shunts are no longer considered as contraindications for surgical ligation because of potential significant revascularization of the intrahepatic portal system [3, 31, 54, 69].
Liver resection or transplantation has been performed for the treatment of extrahepatic or large intrahepatic multifocal shunts not amenable to embolization or in cases of previous failed radiological intervention or where HCC or hepatoblastoma has developed. Shunts with tumors causing obstruction, have a multifocal distribution, are rapidly increasing in size/changing features, or malignancy is suspected require resection with definitive histological diagnosis and potential further treatment. Such tumors may develop at any age even without prior shunt symptomatology [70]. Embolization of HCC is associated with significant ischemic injury (due to the lack of portal vein inflow) and, while very effective, should be used more cautiously. Indication for combined ligation and resection of concomitant malignant liver tumor may also exist in an extrahepatic shunt where resection alone would leave the shunt untreated [41]. Liver transplantation remains the only option for type 1 extrahepatic shunts which maintain increased portomesenteric pressure and fail to establish a sufficient intrahepatic portal network, after temporary shunt occlusion. In some type 1 extrahepatic shunts where radiological occlusion initially seems favorable, “abnormal” cavernous transformation of portal system indicates a nonphysiological remodeling with equivocal results without resolution of symptoms in the longer term. Indications for liver transplant remain poorly defined except for HCC cases [71]. Sakamoto et al. have reviewed 34 patients treated by liver transplantation (both deceased and living donor) for extrahepatic shunts. Indications included hepatopulmonary syndrome, hyperammonemia with encephalopathy and liver tumors. Despite technical difficulties due to abnormal anatomy especially with the portal vein anastomosis, good outcomes were reported, with 31 patients alive (91.2%) at a median follow-up of 18 months [71, 72].
Although overall management may differ among different centers and there is no universal treatment protocol, we are proposing a therapeutic algorithm for all the CPSS which is shown in Figs. 2 and 3. We suggest that all extrahepatic shunts should receive treatment regardless of their symptoms as early intervention has been shown to prevent complications and may preserve intellectual and psychosocial development. For intrahepatic shunts even in the presence of overt symptomatology, observation until 1 year of age should be offered as spontaneous regression may occur. In cases where a congenital shunt is left untreated or not diagnosed until adulthood, indications for treatment depend on the severity of symptoms. First-line treatment currently is endovascular occlusion of the shunt in 1 or 2 stages, reserving surgical options (ligation, resection, transplantation) for those shunts that are not amenable or fail to embolize, or are associated with liver tumors (Figs. 2 and 3).
Conclusion
CPSS are rare embryologic abnormal communications between the portal and systemic venous circulations and are associated with other congenital anomalies. Liver tumors, pulmonary vasculature complications, and hepatic encephalopathy are common. Early recognition and correction with either radiological or surgical occlusion reverses symptoms and prevents long-term complications. Large series with a standard therapeutic approach are not available as yet. Continuing the focus on the pathophysiology and anatomy of these lesions will help guide future management.
Abbreviations
- CPSS:
-
Congenital portosystemic venous shunt
- EPSS:
-
Extrahepatic portosystemic shunt
- HCC:
-
Hepatocellular carcinoma
- IPSS:
-
Intrahepatic portosystemic shunt
References
Kim MJ, Ko JS, Seo JK, Yang HR, Chang JY, Kim GB et al (2012) Clinical features of congenital portosystemic shunt in children. Eur J Pediatr 171(2):395–400
Florio F, Nardella M, Balzano S, Giacobbe A, Perri F (1998) Congenital intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol 21(5):421–424
Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E (2012) Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis 32(4):273–287
Lin ZY, Chen SC, Hsieh MY, Wang CW, Chuang WL, Wang LY (2006) Incidence and clinical significance of spontaneous intrahepatic portosystemic venous shunts detected by sonography in adults without potential cause. J Clin Ultrasound 34(1):22–26
Gitzelmann R, Forster I, Willi UV (1997) Hypergalactosaemia in a newborn: self-limiting intrahepatic portosystemic venous shunt. Eur J Pediatr 156:719–722
Account of two instances of uncommon formation, in the viscera of the human body. By Mr. John Abernethy, Assistant Surgeon to St. Bartholomew’s Hospital. Communicated by Sir Joseph Banks, Bart. P. R. S. Author(s): John Abernethy and Joseph Banks Source: Philosophical Transactions of the Royal Society of London, Vol. 83 (1793), pp. 59–66 Published by: Royal Society Stable
Guérin F, Blanc T, Gauthier F, Abella SF, Branchereau S (2012) Congenital portosystemic vascular malformations. Semin Pediatr Surg 21(3):233–244 Review
Stringer MD (2008) The clinical anatomy of congenital portosystemic venous shunts. Clin Anat 21(2):147–157
Tanoue S, Kiyosue H, Komatsu E, Hori Y, Maeda T, Mori H (2003) Symptomatic intrahepatic portosystemic venous shunt: embolization with an alternative approach. AJR Am J Roentgenol 181(1):71–78
Yu HC, Kim JH, Murakami G, Rodríguez-Vázquez JF, Cho BH (2015) An anomalous portal vein crossing the lesser sac and ending at the upper part of ductus venosus. Anat Cell Biol 48(3):218–221
Senocak E, Oğuz B, Edgüer T, Cila A (2008) Congenital intrahepatic portosystemic shunt with variant inferior right hepatic vein. Diagn Interv Radiol 14:97–99
Alonso-Gamarra E, Parrón M, Pérez A, Prieto C, Hierro L, López-Santamaría M (2011) Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: a comprehensive review. Radiographics 31:707–722
Kim IO, Cheon JE, Kim WS, Chung JW, Yeon KM, Yoo SJ et al (2000) Congenital intrahepatic portohepatic venous shunt: treatment with coil embolisation. Pediatr Radiol 30:336–338
Yoshimoto Y, Shimizu R, Saeki T, Harada T, Sugio Y, Nomura S et al (2004) Patent ductus venosus in children: a case report and review of the literature. J Pediatr Surg 39:E1–E5
Kamimatsuse A, Onitake Y, Kamei N, Tajima G, Sakura N, Sueda T et al (2010) Surgical intervention for patent ductus venosus. Pediatr Surg Int 26:1025–1030
Saito M, Seo Y, Yano Y, Momose K, Hirano H, Yoshida M et al (2013) Successful treatment using coil embolization of a symptomatic intrahepatic portosystemic venous shunt developing through a patent ductus venosus in a noncirrhotic adult. Intern Med 52(5):555–559
Newman B, Feinstein JA, Cohen RA, Feingold B, Kreutzer J, Patel H et al (2010) Congenital extrahepatic portosystemic shunt associated with heterotaxy and polysplenia. Pediatr Radiol 40(7):1222–1230
Takahashi S, Yoshida E, Sakanishi Y, Tohyama N, Ayhan A, Ogawa H (2013) Congenital multiple intrahepatic portosystemic shunt: an autopsy case. Int J Clin Exp Pathol 7:425–431
Lautz TB, Tantemsapya N, Rowell E, Superina RA (2011 Feb) Management and classification of type II congenital portosystemic shunts. J Pediatr Surg 46(2):308–314
Sokollik C, Bandsma RH, Gana JC, van den Heuvel M, Ling SC (2013) Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr 56:675–681
Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J et al (2010) Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr 51:322–330
Papamichail M, Ali A, Quaglia A, Karani J, Heaton N (2016) Liver resection for the treatment of a congenital intrahepatic portosystemic venous shunt. Hepatobiliary Pancreat Dis Int. 15(3):329–333
Park JH, Cha SH, Han JK, Han MC (1990) Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol 155:527–528
Morgan G, Superina R (1994) Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg 29:1239–1241
Howard ER, Davenport M (1997) Congenital extrahepatic portocaval shunts—the Abernethy malformation. J Pediatr Surg 32(3):494–497
Kamata S, Kitayama Y, Usui N, Kuroda S, Nose K, Sawai T et al (2000) Patent ductus venosus with a hypoplastic intrahepatic portal system presenting intrapulmonary shunt: a case treated with banding of the ductus venosus. J Pediatr Surg 35(4):655–657
Takehara Y, Mori K, Edagawa T, Sugimoto M, Takehara H, Ito M et al (2004) Presumed hypoplastic intrahepatic portal system due to patent ductus venosus: importance of direct occlusion test of ductus venosus under open laparotomy. Pediatr Int 46(4):484–486
Stewart JK, Kuo WT, Hovsepian DM, Hofmann LV, Bonham CA, Sze DY. Portal venous remodeling after endovascular reduction of pediatric autogenous portosystemic shunts. J Vasc Interv Radiol 2011;22(8):1199–1205
Kanazawa H, Nosaka S, Miyazaki O, Sakamoto S, Fukuda A, Shigeta T et al (2015) The classification based on intrahepatic portal system for congenital portosystemic shunts. J Pediatr Surg 50(4):688–695 Review
Kobayashi N, Niwa T, Kirikoshi H, Fujita K, Yoneda M, Saito S et al (2010) Clinical classification of congenital extrahepatic portosystemic shunts. Hepatol Res 40(6):585–593
Blanc T, Guerin F, Franchi-Abella S, Jacquemin E, Pariente D, Soubrane O et al (2014) Congenital portosystemic shunts in children: a new anatomical classification correlated with surgical strategy. Ann Surg 260(1):188–198
Grimaldi C, Monti L, Falappa P, d’Ambrosio G, Manca A, de Ville de Goyet J (2012) Congenital intrahepatic portohepatic shunt managed by interventional radiologic occlusion: a case report and literature review. J Pediatr Surg 47:e27–e31
Delle Chiaie L, Neuberger P, Von Kalle T (2008) Congenital intrahepatic portosystemic shunt: prenatal diagnosis and possible influence on fetal growth. Ultrasound Obstet Gynecol 32:233–235
Nagasaka H, Miida T, Yorifuji T, Hirano K, Inui A, Fujisawa T et al (2013) Metabolic improvements in intrahepatic porto-systemic venous shunt presenting various metabolic abnormalities by 4-phenylacetate. Clin Chim Acta 419:52–56
Yamagami T, Nakamura T, Tokiwa K, Ohno K, Itoh H, Maeda T (2000) Intrahepatic portosystemic venous shunt associated with biliary atresia: case report. Pediatr Radiol 30:489–491
Fu L, Wang Q, Wu J, Guo Y, Huang M, Liu T et al (2016 Feb) Congenital extrahepatic portosystemic shunt: an underdiagnosed but treatable cause of hepatopulmonary syndrome. Eur J Pediatr 175(2):195–201
Ohno T, Muneuchi J, Ihara K, Yuge T, Kanaya Y, Yamaki S et al (2008) Pulmonary hypertension in patients with congenital portosystemic venous shunt: a previously unrecognized association. Pediatrics 121:e892–e899
Knirsch W, Benz DC, Bühr P, Quandt D, Weber R, Kellenberger C et al (2016) Catheter interventional treatment of congenital portosystemic venous shunts in childhood. Catheter Cardiovasc Interv 87(7):1281–1292
Pupulim LF, Vullierme MP, Paradis V, Valla D, Terraz S, Vilgrain V (2013) Congenital portosystemic shunts associated with liver tumours. Clin Radiol 68:e362–e369
Kawano S, Hasegawa S, Urushihara N, Okazaki T, Yoshida A, Kusafuka J et al (2007) Hepatoblastoma with congenital absence of the portal vein: a case report. Eur J Pediatr Surg 17(4):292–294
Lautz TB, Shah SA, Superina RA (2016) Hepatoblastoma in children with congenital portosystemic shunts. J Pediatr Gastroenterol Nutr 62(4):542–545
Konstas AA, Digumarthy SR, Avery LL, Wallace KL, Lisovsky M, Misdraji J et al (2011) Congenital portosystemic shunts: imaging findings and clinical presentations in 11 patients. Eur J Radiol 80:175–181
Murray CP, Yoo SJ, Babyn PS (2003) Congenital extrahepatic portosystemic shunts. Pediatr Radiol 33:614–620
Sorkin T, Strautnieks S, Foskett P, Peddu P, Thompson RJ, Heaton N et al (2016) Multiple β-catenin mutations in hepatocellular lesions arising in Abernethy malformation. Hum Pathol 53:153–158
Gong Y, Zhu H, Chen J, Chen Q, Ji M, Pa M et al (2015) Congenital portosystemic shunts with and without gastrointestinal bleeding—case series. Pediatr Radiol 45(13):1964–1971
Singh K, Kapoor A, Kapoor A, Gupta K, Mahajan G (2006) Congenital intrahepatic portosystemic shunt—an incidental rare anomaly. Indian J Pediatr 73:1122–1123
Torigoe M, Maeshima K, Takeshita Y (2013) Congenital intrahepatic portosystemic venous shunt presenting with paraparesis as the initial symptom. Intern Med 52:2439–2442
da Rocha AJ, Braga FT, da Silva CJ, Maia AC Jr, Mourão GS, Gagliardi RJ (2004) Reversal of parkinsonism and portosystemic encephalopathy following embolization of a congenital intrahepatic venous shunt: brain MR imaging and 1H spectroscopic findings. AJNR Am J Neuroradiol 25:1247–1250
Lee SH, Lee DG (2015) Macroscopic hematuria caused by congenital portosystemic shunt and concomitant nutcracker syndrome. Pediatr Int 57(3):e84–e86
Grazioli L, Alberti D, Olivetti L, Rigamonti W, Codazzi F, Matricardi L et al (2000) Congenital absence of portal vein with nodular regenerative hyperplasia of the liver. Eur Radiol 10(5):820–825
Caiulo VA, Presta G, Latini G, Mattioli G, Jasonni V (2001) Diagnosis and follow-up of congenital intrahepatic portosystemic venous shunt by ultrasounds. Acta Paediatr 90:1209–1210
Cho Y, Tokuhara D, Shimono T, Yamamoto A, Higashiyama S, Kotani K et al (2014) Role of per-rectal portal scintigraphy in long-term follow-up of congenital portosystemic shunt. Pediatr Res 75(5):658–662
Córdoba J (2011) New assessment of hepatic encephalopathy. J Hepatol 54:1030–1040
Sharma R, Suddle A, Quaglia A, Peddu P, Karani J, Satyadas T et al (2015) Congenital extrahepatic portosystemic shunt complicated by the development of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 14(5):552–557
Lisovsky M, Konstas AA, Misdraji J (2011) Congenital extrahepatic portosystemic shunts (Abernethy malformation): a histopathologic evaluation. Am J Surg Pathol 35(9):1381–1390
Barton JW 3rd, Keller MS (1989) Liver transplantation for hepatoblastoma in a child with congenital absence of the portal vein. Pediatr Radiol 20:113–114
Turkbey B, Karcaaltincaba M, Demir H, Akcoren Z, Yuce A, Haliloglu M (2006) Multiple hyperplastic nodules in the liver with congenital absence of portal vein: MRI findings. Pediatr Radiol 36:445–448
Palvanov A, Marder RL, Siegel D (2016 Sep) Asymptomatic intrahepatic portosystemic venous shunt: to treat or not to treat? Int J Angiol 25(3):193–198
Paganelli M, Lipsich JE, Sciveres M, Alvarez F (2015) Predisposing factors for spontaneous closure of congenital portosystemic shunts. J Pediatr 167(4):931–935 e12
Yoshimatsu R, Takeuchi Y, Morishita H, Iida N, Okabe H, Yamagami T et al (2006) Successful embolisation of intrahepatic portosystemic venous shunt using coils and n-butyl cyanoacrylate through two approach routes. Br J Radiol 79:e162–e165
Wu L, Zhao L, Lu Y, He L, Hu X (2016) Interventional embolization of congenital intrahepatic shunts in children. Pediatr Radiol 46(4):541–547
Suzuki K, Shimohira M, Hashizume T, Suzuki Y, Shibamoto Y (2013) Dual microcather-dual detachable coil technique in embolization for a congenital intrahepatic portosystemic venous shunt (IPSVS). Minim Invasive Ther Allied Technol 22:316–318
Power AH, Bjarnason H (2012) Large spontaneous intrahepatic portal-systemic venous shunt treated with coil and Amplatzer vascular plug embolization. Perspect Vasc Surg Endovasc Ther 24(2):90–94
Chandrasekharan R, Pullara SK, Thomas T, Kader NP, Moorthy S (2016) Congenital intrahepatic portosystemic shunts: imaging findings and endovascular management. Indian J Radiol Imaging 26(1):92–94
Guneyli S, Cinar C, Bozkaya H, Parildar M, Oran I, Akin Y (2012) Successful transcatheter closure of a congenital high-flow portosystemic venous shunt with the Amplatzer vascular plug II. Perspect Vasc Surg Endovasc Ther 24(4):202–205
Lee SA, Lee YS, Lee KS, Jeon GS (2010) Congenital intrahepatic portosystemic venous shunt and liver mass in a child patient: successful endovascular treatment with an amplatzer vascular plug (AVP). Korean J Radiol 11(5):583–586
Sanada Y, Urahashi T, Ihara Y, Wakiya T, Okada N, Yamada N et al (2012 Mar) The role of operative intervention in management of congenital extrahepatic portosystemic shunt. Surgery 151(3):404–411
Matsuura T, Takahashi Y, Yanagi Y, Yoshimaru K, Yamamura K, Morihana E et al (2016) Surgical strategy according to the anatomical types of congenital portosystemic shunts in children. J Pediatr Surg 51(12):2099–2104
Kimura T, Soh H, Hasegawa T, Sasaki T, Kuroda S, Yuri E et al (2004) Laparoscopic correction of congenital portosystemic shunt in children. Surg Laparosc Endosc Percutan Tech 14(5):285–288
Gordon-Burroughs S, Balogh J, Weiner MA, Monsour HP Jr, Schwartz MR, Gaber AO et al (2014) Liver transplantation in an adult with adenomatosis and congenital absence of the portal vein: a case report. Transplant Proc 46(7):2418–2421
Sakamoto S, Shigeta T, Fukuda A, Tanaka H, Nakazawa A, Nosaka S et al (2012) The role of liver transplantation for congenital extrahepatic portosystemic shunt. Transplantation 93(12):1282–1287
Sakamoto S, Kasahara M, Shigeta T, Fukuda A, Kakiuchi T, Miyasaka M et al (2011) Living donor liver transplantation for multiple intrahepatic portosystemic shunts after involution of infantile hepatic hemangiomas. J Pediatr Surg 46(6):1288–1291
Source of funding
This study is self-funded.
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper. MP made the acquisition, analysis, and interpretation of data and wrote the paper. MEP made the acquisition, analysis, and interpretation of data; created the graphics, and drafted the paper. NH made the conception and design and corrected and revised the article critically. NH conceived the topic, and directed and edited the manuscript. MPa wrote the manuscription draft. MPi has assisted with writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Peter de Winter
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Papamichail, M., Pizanias, M. & Heaton, N. Congenital portosystemic venous shunt. Eur J Pediatr 177, 285–294 (2018). https://doi.org/10.1007/s00431-017-3058-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-3058-x