Abstract
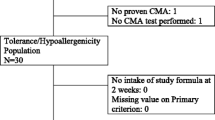
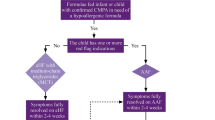
The aim of this study was to investigate the cumulative incidence and predictive variables of treatment failure with a whey-based extensively hydrolyzed formula (w-eHF) in children with cow’s milk allergy (CMA). All children were diagnosed with CMA, using double-blind placebo-controlled food challenge (DBPCFC) with amino acid-based formula as placebo, and receive w-eHF treatment after diagnosis. Forty-nine children with CMA were included. w-eHF treatment failure was defined as incomplete resolution of original CMA symptoms upon w-eHF treatment and disappearance of these symptoms upon replacement of w-eHF with amino acid-based formula. A multiple logistic regression model was used to investigate which variables could predict treatment failure. Twenty-five (51 %; 95 % confidence interval (CI) 38–64 %) of the children with CMA failed on w-eHF. Only “gastrointestinal discomfort” was found to contribute independently to the probability of failing w-eHF, odds ratio (95 % CI) 8.994 (1.007–79.457).
Conclusions: In half of the children with proven CMA, there is incomplete resolution of symptoms upon w-eHF treatment. This study needs to be repeated including DBPCFC with w-eHF to provide more definitive diagnosis, especially since gastrointestinal discomfort seems to be the sole predictive variable for treatment failure. In the meantime, a change in formula should be considered in children with incomplete symptom resolution upon w-eHF treatment.
Similar content being viewed by others
Abbreviations
- AAF:
-
Amino acid-based formula
- AUC:
-
Area under the curve
- c-eHF:
-
Casein-based extensively hydrolyzed formula
- CMA:
-
Cow’s milk allergy
- CMP:
-
Cow’s milk protein
- DBPCFC:
-
Double-blind placebo-controlled food challenge
- e-HF:
-
Extensively hydrolyzed formula
- EuroPrevall BCS:
-
EuroPrevall Birth Cohort Study
- FC:
-
Food challenge
- sIgE:
-
Specific immunoglobulin E
- SPT:
-
Skin prick test
- w-eHF:
-
Whey-based extensively hydrolyzed formula
References
Abell S (2008) Lactose deficiency in infants. Clin Pediatr (Phila) 47:843–844
American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas (2000) Pediatrics 106:346–349
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM (2010) Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol 126:1105–1118
Brand PL, Rijk-van GH (2011) [Cow’s milk allergy in infants: new insights]. Ned Tijdschr Geneeskd 155:A3508
Caffarelli C, Plebani A, Poiesi C, Petroccione T, Spattini A, Cavagni G (2002) Determination of allergenicity to three cow’s milk hydrolysates and an amino acid-derived formula in children with cow’s milk allergy. Clin Exp Allergy 32:74–79
Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, Sundaram V, Paige NM, Towfigh A, Hulley BJ, Shekelle PG (2010) Diagnosing and managing common food allergies: a systematic review. JAMA 303:1848–1856
de Boissieu D, Dupont C (2002) Allergy to extensively hydrolyzed cow’s milk proteins in infants: safety and duration of amino acid-based formula. J Pediatr 141:271–273
De Greef E, Hauser B, Devreker T, Veereman-Wauters G, Vandenplas Y (2012) Diagnosis and management of cow’s milk protein allergy in infants. World J Pediatr 8:19–24
Giampietro PG, Kjellman NI, Oldaeus G, Wouters-Wesseling W, Businco L (2001) Hypoallergenicity of an extensively hydrolyzed whey formula. Pediatr Allergy Immunol 12:83–86
Heyman MB (2006) Lactose intolerance in infants, children, and adolescents. Pediatrics 118:1279–1286
Hill DJ, Cameron DJ, Francis DE, Gonzalez-Andaya AM, Hosking CS (1995) Challenge confirmation of late-onset reactions to extensively hydrolyzed formulas in infants with multiple food protein intolerance. J Allergy Clin Immunol 96:386–394
Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ (2007) The efficacy of amino acid-based formulas in relieving the symptoms of cow’s milk allergy: a systematic review. Clin Exp Allergy 37:808–822
Host A, Halken S (2004) Hypoallergenic formulas–when, to whom and how long: after more than 15 years we know the right indication! Allergy 59(Suppl 78):45–52
Host A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, Bresson JL, Hernell O, Lafeber H, Michaelsen KF, Micheli JL, Rigo J, Weaver L, Heymans H, Strobel S, Vandenplas Y (1999) Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child 81:80–84
Keil T, McBride D, Grimshaw K, Niggemann B, Xepapadaki P, Zannikos K, Sigurdardottir ST, Clausen M, Reche M, Pascual C, Stanczyk AP, Kowalski ML, Dubakiene R, Drasutiene G, Roberts G, Schoemaker AF, Sprikkelman AB, Fiocchi A, Martelli A, Dufour S, Hourihane J, Kulig M, Wjst M, Yazdanbakhsh M, Szepfalusi Z, van Ree R, Willich SN, Wahn U, Mills EN, Beyer K (2010) The multinational birth cohort of EuroPrevall: background, aims and methods. Allergy 65:482–490
Klemola T, Vanto T, Juntunen-Backman K, Kalimo K, Korpela R, Varjonen E (2002) Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: a prospective, randomized study with a follow-up to the age of 2 years. J Pediatr 140:219–224
Kneepkens CMF (2005) Drongelen KIv, Aarsen CJE: National Guideline Food Allergy in Infants ("Landelijke standaard voedselallergie bij zuigelingen")
Kneepkens CM, Meijer Y (2009) Clinical practice. Diagnosis and treatment of cow’s milk allergy. Eur J Pediatr 168:891–896
Knipping K, van Esch BC, van Ieperen-van Dijk AG, van Hoffen E, van Baalen T, Knippels LM, van der Heide S, Dubois AE, Garssen J, Knol EF (2012) Enzymatic treatment of whey proteins in cow’s milk results in differential inhibition of IgE-mediated mast cell activation compared to T-cell activation. Int Arch Allergy Immunol 159:263–270
McBride D, Keil T, Grabenhenrich L, Dubakiene R, Drasutiene G, Fiocchi A, Dahdah L, Sprikkelman AB, Schoemaker AA, Roberts G, Grimshaw K, Kowalski ML, Stanczyk-Przyluska A, Sigurdardottir S, Clausen M, Papadopoulos NG, Mitsias D, Rosenfeld L, Reche M, Pascual C, Reich A, Hourihane J, Wahn U, Mills EN, Mackie A, Beyer K (2012) The EuroPrevall birth cohort study on food allergy: baseline characteristics of 12,000 newborns and their families from nine European countries. Pediatr Allergy Immunol 23:230–239
Dutch Pediatric Society: diagnosing cow milk allergy in children in the Netherlands (Richtlijn diagnostiek van koemelkallergie bij kinderen in Nederland); 2012. (www.nvk.nl)
Sampson HA, James JM, Bernhisel-Broadbent J (1992) Safety of an amino acid-derived infant formula in children allergic to cow milk. Pediatrics 90:463–465
Sicherer SH, Noone SA, Koerner CB, Christie L, Burks AW, Sampson HA (2001) Hypoallergenicity and efficacy of an amino acid-based formula in children with cow’s milk and multiple food hypersensitivities. J Pediatr 138:688–693
Terracciano L, Schunemann H, Brozek J, Agostoni C, Fiocchi A (2012) How DRACMA changes clinical decision for the individual patient in CMA therapy. Curr Opin Allergy Clin Immunol 12:316–322
Twisk JWR (2010) Multiple regressieanalyse: associatiemodellen en predictiemodellen. In: Twisk JWR (ed) Inleiding in de toegepaste biostatistiek. Elsevier, Gezondheidszorg, pp 229–274
Venter C, Brown T, Shah N, Walsh J, Fox AT (2013) Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy—a UK primary care practical guide. Clin Transl Allergy 3:23
Acknowledgment
The authors would like to thank all children and their parents for participating in the Dutch EuroPrevall Birth Cohort Study. We thank the nurses of the Emma Children’s Hospital department for day care for their support in performing the food challenges and the employees of the special kitchen for preparing all the challenges. Furthermore, we would like to thank Dr. JH van der Lee, I de Vreede, N Rutjes, and L Hulshof for their valuable scientific input.
Funding source
The EuroPrevall study is funded by the European Commission (FOOD-CT_2005-514000). The Dutch cohort received unrestricted grants from Nutricia, the Netherlands, Nutricia Advanced Medical Nutrition, the Netherlands, AstraZeneca, the Netherlands, TEVA, the Netherlands, and GlaxoSmithKline, the Netherlands. These companies were not involved in the design of the study or collecting the data.
Conflict of interest
Aline B Sprikkelman received speaker’s fees from Nutricia Advanced Medical Nutrition, The Netherlands, and Mead Johnson, The Netherlands, and research grants from Danone Research, The Netherlands. All other authors declare no potential conflict of interest.
Ethical statement
The Medical Ethics Committee of the Academic Medical Hospital (METC 06/005) approved the Dutch EuroPrevall BCS. Written informed consent was obtained from both parents of each child, unless only one of them had parental rights.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Nadal
Nicole C. M. Petrus and Anne-Fleur A. Schoemaker contributed equally.
Rights and permissions
About this article
Cite this article
Petrus, N.C.M., Schoemaker, AF.A., van Hoek, M.W. et al. Remaining symptoms in half the children treated for milk allergy. Eur J Pediatr 174, 759–765 (2015). https://doi.org/10.1007/s00431-014-2456-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-014-2456-6