Abstract
Despite high vaccination coverage, over the last fifteen years there has been a worldwide resurgence of B. pertussis infection. While classical pertussis in the prevaccine era was primarily a childhood disease, today with widespread vaccination, there has been a shift in the incidence of disease to adolescents and adults. Centers of Disease Control and Prevention (CDC) data from 2004 reveal a nearly 19-fold increase in the number of cases in individuals 10–19 years and a 16-fold increase in persons over 20 years. Indeed adolescent and adults play a significant role in the transmission of pertussis to neonates and infants who are vulnerable to substantial morbidity and mortality from pertussis infection. Several explanations have been proposed to explain the increasing incidence of disease, with waning immunity after natural infection or immunization being widely cited as a significant factor. Improving molecular biology diagnostic techniques, namely PCR assays, also accounts for the increasing laboratory diagnosis of pertussis. Expanding vaccination strategies including universal immunization of adolescents, targeted immunization of adults, and in particular, healthcare workers, childcare providers and parents of newborns, will likely improve pertussis control. With pertussis continuing to pose a serious threat to infants, and greatly affecting adolescents and adults, there remains a need to: (a) increase the awareness of physicians as to the growing pertussis problem, (b) standardize diagnostic techniques, and (c) implement various new vaccine strategies to enhance its control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pertussis, an acute infectious illness of the respiratory tract remains endemic in developed nations despite high vaccination coverage [7, 8, 16]. While the early use of whole-cell vaccine was highly effective in reducing the incidence of reported pertussis in the United States in the 1970s, there has been a resurgence of reported pertussis over the last 15 years [8, 16, 31]. Worldwide, there are an estimated 50 million cases occurring annually (90% of which are in developing countries), and there are as many as 400,0000 pertussis-related deaths [16, 31]. It is general consensus, moreover, that the reported incidence of pertussis is considerably lower than its actual incidence [8, 31].
Though in the prevaccine era pertussis was regarded as a childhood disease affecting primarily young children, pertussis epidemiology in the postvaccine era is different [8, 17]. Infants are the most vulnerable group with the highest rates of complications and mortality, yet adolescents and adults now comprise a significant percentage of cases and a conduit of infection for the infants [8, 13, 17].
PCR, culture, and serology are the mainstay of the laboratory diagnosis of pertussis, with various factors affecting the sensitivity and specificity of each modality [8, 24, 36]. However, in recent years, PCR has become an increasingly more popular tool and has significantly contributed to the increasing laboratory diagnosis of pertussis [8, 27, 36].
Advances have also been made with regard to prevention and disease control, with experts from 17 countries recently establishing the Global Pertussis Initiative (GPI) with the aim of analyzing the status of pertussis and enhancing existing immunization strategies [8, 15].
Epidemiology of pertussis
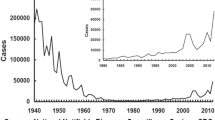
Before the introduction of the whole-cell pertussis vaccine in the1940s, there were approximately 200,000 cases reported annually in the US [37]. Immunizations reduced disease rates and in 1976 pertussis incidence reached a nadir of 1,010 reported cases [13, 37]. However, since that time, there has been a substantial increase in the number of cases reported [8, 13, 31]. Indeed, over the past 15 years, there has been a marked increase in the incidence of pertussis with reported disease in the US reaching a rate of 8.9 per 100,000 in 2004 with nearly 19,000 provisional reported cases [7, 17]. It is also well established that, despite high vaccination coverage for primary immunization in infants and children, pertussis continues to be a global concern with increased incidence in many countries including Argentina, Australia, Canada, Italy, Japan, the Netherlands, Switzerland and the US [31]. It is also widely noted that in recent years there is a general shift in the age distribution of pertussis, with adults and adolescents an underrecognized but significant source of infection for neonates and infants [8, 13, 15–17]. Data from the EUVAC-NET project, a network for the epidemiologic surveillance and control of communicable diseases in the European community, demonstrate that between 1998 and 2002, the rate of disease incidence remained stable at a high rate among children less than 1 year old. Nevertheless, these data indicate that the incidence rate among adults doubled in 5 years [4]. Similarly, Centers for Disease Control and Prevention (CDC) surveillance data from 1990–2003 demonstrate that the reported incidence of pertussis among adolescents has substantially increased with a nearly ten-fold rise [17]. Moreover, when comparing pertussis disease rates in 1990–1993, recent CDC data from 2004 reveal a nearly 19-fold increase in the number of cases in persons aged 10–19 years and a 16-fold increase in persons over 20 years [13].
Several factors have been proposed as underlying the increasing incidence of pertussis disease including waning immunity with subsequent atypical disease manifestations, increasing awareness by public health personnel with subsequent enhanced surveillance and improved laboratory diagnostics [8, 17, 31].
Waning of both vaccine-induced immunity and infection-acquired immunity is widely cited as an important reason for recent epidemiologic trends [7, 8, 36, 37]. While the assessment of the duration of immunity afforded after either natural infection or vaccination is complex, individuals are clearly susceptible to initial infection/reinfection after vaccination or previous pertussis illness, respectively. Studies vary in their estimation of protection against disease with protective immunity after natural infection waning 7–20 years after illness, and duration of immunity after vaccination waning at approximately 4–12 years in children [36]. Yet, regardless of the precise interval, when individuals do contract pertussis after the waning of their immunity, their disease manifestations are frequently atypical [8, 17, 27]. As such, their illness is often underdiagnosed. Such underdiagnosis poses a potentially serious public-health concern in that those untreated persons with protracted cough continue to unknowingly transmit the disease to others.
Finally, it has been proposed that the increased incidence rates may also be a function of enhanced surveillance as well as improved and more sensitive diagnostic lab techniques (e.g., PCR), in that such techniques allow for the diagnosis of cases that would probably have been missed in the past [8, 17, 27, 35]. Nevertheless, it is important to note, that the current estimates are likely to be, if anything, an underrepresentation of the true incidence of disease [8, 31]. First, the clinical diagnosis of pertussis is complicated by underconsulting, particularly among adolescents and adults [8]. Second, with prolonged cough often being their only clinical feature, by the time these adolescents and adults finally do seek medical attention, it is often too late to culture or detect the organism by PCR, thus potentially resulting in a missed diagnosis [8, 17, 31, 35]. Moreover, the wide heterogeneity in disease expression, modification of disease by immunization, mixed infection, inconsistent definition, and insensitive nonstandardized, poorly performed, or lack of available laboratory tests, further complicate physician diagnosis [8]. While the classic or “typical” pertussis may be easily recognized, it is seen less often since general immunization began. Instead, atypical pertussis, usually characterized by the absence of whoop and often a somewhat shorter duration of cough, is more common than classical pertussis among adolescents and adults [8, 17]. And finally, it should be noted that, immunized young children that are PCR positive for B pertussis can be asymptomatic [29, 31].
Regardless of whether an individual displays classical pertussis signs and symptoms or a more protracted, atypical cough, pertussis may not be suspected because of the misconception among many physicians that pertussis is a childhood disease [8, 17]. Co-occurrence of other infections like Influenza A or B, adenovirus, and RSV may also complicate the clinical diagnosis [8]. And, finally, even when diagnosed, pertussis is often under reported [8]. Indeed, Sutter and Cochi report that in the US, only an estimated 11.6% of pertussis cases were actually reported [17, 30]. Thus multiple institutional, clinical, and laboratory factors diminish the true assessment of pertussis incidence, and the current data clearly are an underestimation of the true burden of disease.
Laboratory diagnosis of pertussis
Because accurate diagnosis of pertussis cannot be made by clinical signs and symptoms alone, there is a need for improved laboratory diagnosis of pertussis [17]. While several laboratory techniques exist for the identification of B. pertussis, namely, culture, serology and PCR, overall, several practical factors may adversely affect the sensitivity of its laboratory diagnosis. Delayed specimen collection, poor specimen collection techniques, specimen transport problems, and lab media contamination are but a few of the practical constraints often influencing the outcomes of the laboratory diagnosis of pertussis. Moreover, previous exposure to the organism, patient’s age, stage of disease, previous antibiotic administration, and immunization are other factors that may have a substantial impact on the sensitivity of the tests. Finally, limited access to diagnostic or laboratory methods, in both developed and developing countries, undoubtedly affects B. pertussis laboratory confirmation [8, 24].
Culture
B. pertussis is a fastidious gram-negative cocobacillus, and its isolation from nasopharyngeal secretions remains the gold standard for diagnosis. Culture requires collection of a posterior nasopharyngeal specimen with a dacron or calcium alginate swab. To increase the yield of positive cultures, specimens should be immediately plated onto selective Regan Lowe agar or Bordet Gengou medium, selective media that are seldom readily available in physician's offices because of their cost and short shelf-life [17, 24]. The main reasons for failure of bacterial growth in culture, from correctly collected and transported specimens, stem from bacterial and fungal contamination and the lack of fresh media [24]. Generally, 7–10 days are required to grow, isolate, and identify the organism, an obvious limitation of the culture method.
The timing of obtaining specimens for culture is also of paramount importance and greatly affects its yield. The proportion of patients testing positive for pertussis by culture is highest when the initial specimens are obtained early in the course of illness, i.e., during the early catarrhal phase of the illness when the organism is present in the nasopharynx in sufficient quantity. However, adults and adolescents often present late in the course of their illness, thereby greatly reducing the likelihood of culturing the organism [17, 24]. Studies also demonstrate that proportions of positive cultures decline in patients who have been previously immunized and undoubtedly in those in whom antibiotics have been started. Thus, given the limited “window of opportunity” for positive culture, it is important to stress that a negative culture does not exclude pertussis [16]. Finally, it is important to emphasize, that despite its low yield, culture should be attempted, as the bacterial isolates are needed for genotypic and phenotypic analysis.
PCR
The use of PCR for the diagnosis of pertussis is rapidly evolving as it provides a sensitive, rapid means for laboratory diagnosis in circumstances in which the probability of a positive culture is low [8, 17, 32, 35]. Notably, the CDC and World Health Organization (WHO) now include a positive PCR in their lab definition of pertussis [17]. While the sensitivity of PCR also decreases somewhat with the duration of cough and among previously immunized individuals, it is nevertheless a significantly more robust tool for diagnosis for those in the later stages of the disease or for those who have already received antibiotics [17, 35]. Specifically, in their 2005 consensus paper, the European Research Programme for Improved Pertussis Strain Characterization and Surveillance (EUpertstrain) state that the real-time PCR is more sensitive than culture for the detection of B. pertussis, especially after the first 3–4 weeks of coughing and after antibiotic therapy has been initiated [16, 27]. In a prospective study in which nasopharyngeal samples were obtained simultaneously for both PCR and culture, the identification of B. pertussis infections was nearly four-fold higher with PCR [8, 28]. Finally, PCR is an invaluable tool for the diagnosis of pertussis among young infants since the yield of culture is low and serology is problematic in this age group [1, 17].
As with culture, important factors for the successful application of PCR in the diagnosis of infection by Bordetella species include proper sample collection and preparation. For example, a Dacron swab with a fine flexible wire shaft, and not calcium alginate, is the recommended swab. After obtaining the nasopharyngeal sample, the swab should be shaken vigorously in saline solution, the swab discarded and the vial sealed for further processing [24]. Appropriate primer selection, amplification conditions, and controls are also essential for effective PCR testing. Primers have been derived from four chromosomal regions and common primers employed in PCR detection systems include IS481, IS1001 PTp1, and PTp2 [8, 24]. Inherent with the high sensitivity, false positive results are a well-recognized problem associated with the PCR diagnosis of pertussis and other respiratory illnesses. While at the present time, PCR is not routinely available and its methods need more standardization, optimization, and quality control, in the future, an internationally accepted standardized kit might be available, which would facilitate the expanded use of PCR for pertussis diagnosis [16, 35].
Serology
Natural infection with B. pertussis is followed by an increase in serum levels of IgA, IgM, and IgG antibodies to specific pertussis antigen whereas the primary immunization of children induces mainly IgM and IgG antibodies. During the past 15 years, ELISAs have constituted the mainstay of serologic diagnosis using specific B. pertussis protein as antigens, and the serologic diagnosis of pertussis is suspected with increases in IgA or IgG antibody titers to pertussis toxin (PT), filamentous hemmaglutinin (FHA), pertactin, fimbriae or sonicated whole organisms in two serum samples collected 2–4 weeks apart [24]. Notably, these antibody responses to FHA are not specific to B. pertussis, but also occur following other Bordetella species; moreover, these antibodies may be cross-reacting epitopes to other bacteria including H. influenzae and M. pneumoniae. Thus, the greatest sensitivity and specificity for the serological diagnosis of B. pertussis infection is by ELISA measurement of IgG and IgA antibodies to PT demonstrating at least a two-fold rise in titer between acute- and convalescent-phase sera [24].
Still, the main problem in the serologic diagnosis of B. pertussis by ELISA is the frequent delay in obtaining the acute-phase specimen. In individuals with re-infections, there is a rapid increase in titer such that if a “delayed” acute-phase sample is obtained, the titer is likely to have already peaked, thereby hampering the detection of the significant titer increase between the acute- and convalescent-phase serum samples [24]. Notably, for those individuals not recently immunized, a single-serum sample ELISA may circumvent the problem, as ill patients will have significantly higher ELISA titers than the geometric mean titers (GMT) of healthy controls [23–25]. With this in mind, although a rise in PT IgA is more suggestive of a recent antibody response, it is less consistent than a PT IgG rise; hence, in adolescents and adults, a single high value of IgG or IgA antibodies to PT suggests pertussis infection [17, 24, 35]. Indeed, de Melker et al. demonstrated that an IgG concentration to PT of at least 100 units/mL in a single serum sample was diagnostic of either a recent or active pertussis infection [10].
The serological diagnosis of pertussis among infants also has notable limitations. Some culture-positive patients, particularly infants younger than 3 months, do not develop measurable antibodies, a finding that calls into question the utility of even obtaining a serum specimen for serology in young infants [8].
In summary, despite the shortcomings of serology, a single-sample serology test can be a useful tool, particularly among older patients presenting late in the course of their illness when culture and PCR testing are negative.
Use of antibiotics in the treatment and prevention of pertussis
Antimicrobial agents administered early in the course of disease, i.e., during the catarrhal stage, may ameliorate the disease; although, after the cough is established antibiotics do not have a discernable effect on the course of the illness, but rather are recommended to limit the spread of organisms to other individuals [9].
Erythromycin, clarithromycin or azithromycin are now considered first-line agents for treatment (and prophylaxis) of pertussis in individuals 6 months of age or older (Table 1). The antibiotic choice for infants younger than 6 months of age, however, requires special attention. The FDA has not yet approved azithromycin or clarithromycin for use in infants younger than 6 months; however, the 2006 AAP endorsed Red Book lists azithromycin as the preferred macrolide for this age group because of the risk of idiopathic hypertrophic pyloric stenosis associated with erythromycin [9]. Notably however, there was a recent report of infantile hypertrophic pyloric stenosis among two young infants treated with azithromycin for pertussis [26].
Postexposure prophylaxis
The American Academy of Pediatrics’ 2006 Red Book recommends that chemoprophylaxis be administered to all household contacts and other close contacts, regardless of age and immunization status. The rationale behind this recommendation is that administration of chemoprophylaxis to asymptomatic contacts within 21 days of onset of cough in the index patient can limit secondary transmission [9]. Other countries like the United Kingdom limit the use of prophylaxis for the protection of only those with the greatest risk from pertussis, namely, young infants [11, 33]. Notably, an evidence-based review of literature on the use of erythromycin in preventing secondary transmission of pertussis to close contacts concluded that in countries where effective pertussis vaccines are in use, chemoprophyalxis should be limited to those most susceptible to the complications of pertussis (i.e., unimmunized or partially immunized infants) and to those individuals who come in close contact with the latter [11, 12]. Regardless of the policy, the agents, dose, and duration of prophylaxis are the same as for treatment of pertussis.
Prevention of pertussis: vaccination strategies
Pertussis vaccines licensed for use in infants, children, and adults vary across countries. These vaccines differ both in terms of their active ingredients and in terms of the other diseases for which coverage is provided (e.g., polio, diptheria). For example, Repevax (Sanofi Pasteur) contains diptheria, tetanus, pertussis (acellular, component) as well as inactivated polio, whereas ADACEL contains only tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Vaccination strategies similarly vary from country to country. Over the last several years, many potential immunization strategies have been proposed to improve pertussis control (Table 2). Universal immunization of adolescents and adults, selective perinatal immunization of women who recently gave birth, and close contacts of newborns, are but a few of the strategies that were discussed by the Global Pertussis Initiative (GPI), which first convened in 2001. Specified immunization goals also included improvement of current infant and toddler vaccination programs [15]. The second GPI convened in 2005 and reiterated several intervention strategies to address the ongoing severe pertussis disease among neonates and infants [16].
Immunization of adolescents
As previously noted, the incidence of pertussis among adolescents is increasing and these individuals then serve as a reservoir of infection to unvaccinated or incompletely vaccinated infants [13]. Two Tdap vaccines (Boostrix and ADACEL) are licensed for use in the US. Recently, the CDCs Advisory Committee on Immunization Practices (ACIP) has recommended routine Tdap for adolescents from 11–18 years [3, 13, 16]. Several other countries including Canada, Austria, Australia, France, and Germany have also introduced the universal immunization of adolescents [16]. In Germany, for example, the current immunization schedule recommends DTaP at 2, 3, 4, and 11–14 months, and dTaP at 5–6 years and at 9–17 years [14]. For a complete overview of the pertussis vaccination in other European countries please access the EUVAC.NET website [14]. Future studies will be needed to evaluate the duration of protection afforded and the potential need for an adult booster.
Immunization of adults
The Adult Pertussis Trial (APERT), a study sponsored by the United States’ National Institute of Health (NIH), has recently demonstrated the efficacy of acellular pertussis vaccines in preventing pertussis disease in adults (and adolescents). [16, 20, 21, 34]. To date, only ADACEL is licensed for use in adults, and the recommended adult immunization schedule in the US (October 2006–September 2007) now recommends that Tdap replace a single dose of Td for adults <65 years who have not previously received a dose of Tdap (either in the primary series, booster or for wound management) [6]. Given the increased public awareness of adolescent and adult pertussis, in conjunction with perhaps an increased awareness about vaccines in general (e.g., HPV and influenza), the general public may be more receptive to a universal adult vaccination against pertussis [16]. The expected benefits of such programs would be to build up herd immunity and reduce disease. Alternatively, the selective vaccination of only those adults at highest risk of transmitting B. pertussis to vulnerable infants is likely to decrease both the incidence and the impact of pertussis on young infants. Regardless of the approach used, successful adult vaccination programs must include education and public awareness.
Cocoon strategy
The vaccination of household members, including parents and siblings of newborn infants, has been recently coined the cocoon strategy [16]. Recent studies have demonstrated that parents are frequently the source of pertussis infection to their infants [2, 13, 16, 19, 22]. While implementation of this strategy is expected to lead to only modest reductions in typical adult cases, there is a strong indirect effect on infants and young children. In countries where universal immunization of adults is not yet feasible, many experts consider such targeted immunization as “worthy of implementation” [16]. Presently, the cocoon strategy is recommended in several European countries, including Australia, France, Germany, and Austria [16].
Maternal vaccination
Although there is efficient placental transfer of pertussis antibodies, low maternal levels and rapid decay in newborns render the infants vulnerable to life-threatening pertussis [16, 18]. Maternal immunization during pregnancy might afford some degree of protection to mother and infant during a vulnerable period, and the use of Tdap during pregnancy is currently under consideration.
Neonatal vaccination
Given the resurgence of reported pertussis in infant populations noted in multiple countries, and the high morbidity and mortality in this age group, newborn pertussis immunization is a potentially attractive strategy [5, 16, 19]. It is still unclear, however, if such a strategy will induce sufficient and timely immunity for this targeted immunization. Future trials are needed to address these concerns.
Conclusion
Despite the increasing awareness of B. pertussis, it continues to affect millions of people worldwide. While classical pertussis was once regarded as a “child’s disease”, today, pertussis poses a serious threat to infants, and greatly affects adolescents adults, now functioning as reservoirs of infection. While advances in molecular biology have undoubtedly increased the capacity to diagnose pertussis, work is still needed to standardize laboratory techniques. The increased awareness of the pertussis problem among experts and the lay public will hopefully pave the way for the implementation of various vaccine strategies to enhance its control.
Abbreviations
- ACIP:
-
Advisory Committee on Immunization Practices
- APERT:
-
Adult Pertussis Trial
- CDC:
-
Centers of Disease Control and Prevention
- Eupertstrain:
-
European research programme for improved pertussis strain characterization and surveillance
- FHA:
-
Filamentous hemmaglutinin
- GMT:
-
Geometric mean titer
- GPI:
-
Global Pertussis Initiative
- NIH:
-
National Institute of Health
- PCR:
-
Polymerase chain reaction
- PT:
-
Pertussis toxin
- DTaP:
-
Diphtheria vaccine (normal dose), tetanus vaccine (normal dose), acellular pertussis vaccine (normal dose)
- DtaP:
-
Diphtheria vaccine (normal dose), low dose tetanus vaccine (booster dose), acellular pertussis vaccine (normal dose)
- Tdap:
-
Tetanus vaccine (normal dose), low dose diphtheria vaccine (booster dose), low dose pertussis acellular vaccine (booster dose)
- Td:
-
Tetanus vaccine (normal dose), low dose diphtheria vaccine (booster dose)
- WHO:
-
World Health Organization
References
Bamberger E, Lahat N, Gershtein V, Gershtein R, Benilevi D, Shapiro S, Kassis I, Rubin L, Srugo I (2005) Diagnosing pertussis: the role of polymerase chain reaction. Isr Med Assoc J 7(6):397–399
Bamberger E, Starets-Haham O, Greenberg D, Karidis A, Porat N, Bar-Joseph G, Gershtein R, Srugo I (2006) Adult pertussis is hazardous for the newborn. Infect Control Hosp Epidemiol 27(6):623–625
Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BS, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, Srivastava PU, Moran JS, Schwartz B, Murphy TV (2006) Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. Morb Mortal Wkly Rep 55(RR03):1–34
Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE (2005) Resurgence of pertussis in Europe. Pediatr Infect Dis J 24:761–765
Centers for Disease Control and Prevention (2005) Pertussis- United States, 2001–2003. MMWR Morb Mortal Wkly Rep 54(50):1283–1286
Centers for Disease Control and Prevention (2006) Recommended adult immunization schedule United States, October 2006–September 2007. Centers for Disease Control and Prevention. http://www.cdc.gov/nip/recs/adult-schedule.pdf. Cited 22 May 2007
Cherry JD (2006) Epidemiology of pertussis. Pediatr Infect Dis J 25:361–362
Cherry JD, Grimprel E, Guiso N, Heininger U, Mertsola J (2005) Defining pertussis epidemiology clinical, microbiologic and serologic perspectives. Pediatr Infect Dis J 24:S25–S34
Committee on Infectious Diseases, American Academy of Pediatrics (2006) Pertussis. In: Pickering LK, Baker CJ, Long SS, McMillan JA (eds) Red book: report of the committee on infectious diseases, 27th edn. Elk Grove Village, IL, pp 498–520
de Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, Elvers LH, Berbers GA, van Der Zee A, Schellenkens JF (2000) Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol 38(2):800–806
Dodhia H, Crowcroft NS, Bramley JC, Miller E (2002) UK guidelines for use of erythromycin chemoprophylaxis in persons exposed to pertussis. J Public Health Med 24:200–206
Dodhia H, Miller E (1998) Review of the evidence for the use of erythromycin in the management of persons exposed to pertussis. Epidemiol Infect 120:143–149
Edwards K, Freeman DM (2006) Adolescent and adult pertussis: disease burden and prevention. Curr Opin Pediatr 18:77–80
EUVAC.NET (1999) German immunization schedule. http://www.euvac.net/graphics/euvac/vaccination/germany.html. Cited 22 May 2007
Forsyth K, Tan T, von König CH, Caro JJ, Plotkin S (2005) Potential strategies to reduce the burden of pertussis. Pediatr Infect Dis J 24:S69–S74
Forsyth KD, von König CH, Tan T, Caro J, Plotkin S (2007) Prevention of pertussis: recommendations derived from the second Global Pertussis Initiative roundtable meeting. Vaccine (in press). DOI 10.1016/j.vaccine.2006.12.017
Greenberg DP (2005) Pertussis in adolescents increasing incidence brings attention to the need for booster immunization of adolescents. Pediatr Infect Dis J 24:721–728
Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ (2004) Prevalence of pertussis antibodies in maternal delivery cord and infant serum. J Infect Dis 190:335–340
Kowalzik F, Barbosa AP, Fernandes VR, Carvalho RP, Avila-Aguero ML, Goh DYT, Goh A, de Miguel MJG, Moraga F, Roca J, Campins M, Huang LM, Quian J, Riley N, Beck D, Verstraeten T (2007) Prospective multinational study of pertussis infection in hospitalized infants and their household contacts. Pediatr Infect Dis J 25:238–242
Kretsinger K, Broder KR, Coretese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, Mijalski CM, Brown KH, Murphy TV (2006) Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid reduced diphtheria toxoid and acellular pertussis vaccine morb mortal. Wkly Rep 55(RR17):1–33
Le T, Cherry JD, Chang SJ, Knoll MD, Lee ML, Barenkamp S, Bernstein D, Edelman R, Edwards KM, Greenberg D, Keitel W, Treanor J, Ward JI, APERT Study (2004) Immune responses and antibody decay after immunization of adolescents and adults with an acelluar pertussis vaccine: the APERT Study. J Infec Dis 190:535–544
Long SS, Welkon CJ, Clark JL (1990) Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infec Dis 161:473–479
Marchant CD, Loughlin AM, Lett SM, Todd CW, Wetterlow LH, Biccieri R, Higham S, Etkind P, Silva E, Siber GR (1994) Pertussis in Massachusetts 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis 169:1297–1305
Mattoo S, Cherry JD (2005) Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18:326–382
Mink CM, Cherry JD, Christenson P, Lewis K, Pineda E, Shlian D, Dawson JA, Blumberg DA (1992) A search for Bordetella pertussis infection in university students. Clin Infect Dis 14:464–471
Morrison W (2007) Infantile hypertrophic pyloric stenosis in infants treated with azithromycin (report). Pediatr Infect Dis J 26(2):186–188
Riffelmann M, von König CH, Caro V, Guiso N (2005) Nucleic acid amplification tests for diagnosis of Bordetella infections. J Clin Microbiol 43:4925–4929
Schläpfer G, Cherry JD, Heininger U, Uberall M, Schmitt-Grohe S, Laussucq S, Just M, Stehr K (1995) Polymerase chain reaction identification of Bordetella pertussis infections in vaccines and family members in a pertussis vaccine efficacy trial in Germany. Pediatr Infect Dis J 14:209–214
Srugo I, Benilevi D, Madeb R, Shapiro S, Shohat T, Somekh E, Rimmar Y, Gershtein V, Gershtein R, Marva E, Lahat N (2000) Pertussis infection in fully vaccinated children in day-care centers, Israel. Emerg Infect Dis 6(5):526–529
Sutter RW, Cochi SL (1992) Pertussis hospitalizations and mortality in the United States, 1985–1988. Evaluation of the completeness of national reporting. JAMA 267:386–391
Tan T, Trindade E, Skowronski D (2005) Epidemiology of pertussis. Pediatr Infect Dis J 24:S10–S18
van Kruijssen AM, Templeton KE, van der Plas RN, van Doorn R, Claas EC, Sukhai RM, Kuijper EJ (2006) Detection of respiratory pathogens by real-time PCR in children with clinical suspicion of pertussis. Eur J Pediatr. Online First. DOI 10.1007/s00431-006-0378-7
von König CH (2005) Use of antibiotics in the prevention and treatment of pertussis. Pediatr Infect Dis J 24:S66–S68
Ward JI, Cherry JD, Chang SJ, Partridge S, Lee H, Treanor J, Greenberg DP, Keitel W, Barenkamp S, Bernstein DI, Edelman R, Edwards K, APERT Study Group (2005) Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med 353:1555–1563
Wendelboe AM, Van Rie A (2006) Diagnosis of pertussis: a historical review and recent developments. Expert Rev Mol Diagn 6:857–864
Wendelboe AM, Van Rie A, Salmaso S, Englund J (2005) Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 24:S58–S61
Wheeler JG, Simmons Al (2005) Pertussis update. Pediatr Infect Dis J 24:829–830
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bamberger, E.S., Srugo, I. What is new in pertussis?. Eur J Pediatr 167, 133–139 (2008). https://doi.org/10.1007/s00431-007-0548-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-007-0548-2