Abstract
Tetraspanins are membrane organizing proteins that play a role in organizing the cell surface through the formation of subcellular domains consisting of tetraspanins and their partner proteins. These complexes are referred to as tetraspanin enriched microdomains (TEMs) or the tetraspanin web. The formation of TEMs allows for the regulation of a variety of cellular processes such as adhesion, migration, signaling, and cell fusion. Tetraspanin CD53 is a member of the tetraspanin superfamily expressed exclusively within the immune compartment. Amongst others, B cells, CD4+ T cells, CD8+ T cells, dendritic cells, macrophages, and natural killer cells have all been found to express high levels of this protein on their surface. Almost three decades ago it was reported that patients who lacked CD53 suffered from an increased susceptibility to pathogens resulting in the clinical manifestation of recurrent viral, bacterial, and fungal infections. This clearly suggests a vital and non-redundant role for CD53 in immune function. Yet, despite this striking finding, the specific functional roles of CD53 within the immune system have remained elusive. This review aims to provide a concise overview of the published literature concerning CD53 and reflect on the underappreciated role of this protein in immune cell regulation and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: tetraspanins in the immune system
There are 33 known tetraspanin family members expressed in human cells. Tetraspanins are integral membrane proteins containing four transmembrane domains, two extracellular domains, and two short intracellular tails [1]. Tetraspanins are approximately 20–25 kDa in size and defined by the presence of four, six or eight conserved cysteine domains found within the second extracellular loop (EC2) [2]. Tetraspanins are membrane organizing proteins that form microdomains by exploiting their ability to interact in cis with each other and with partner proteins located either on the cell surface or on intracellular membranes. Specific α-helices located within the EC2 exhibit high variability allowing the different tetraspanins to interact specifically with certain partner proteins. Through these interactions they are able to organize proteins into membrane microdomains referred to as tetraspanin enriched microdomains (TEMs) or the tetraspanin web [3]. TEMs bring together functionally related proteins to regulate different cellular processes, including adhesion, migration, signaling, activation, and cell fusion [4,5,6,7]. Well-characterized tetraspanin partners include integrins, co-stimulatory molecules, and protein kinase C (PKC) [6, 8, 9]. Though the majority of tetraspanins have a ubiquitous expression pattern, there are two tetraspanins which are known to be restricted to the immune compartment, namely, CD37 and CD53. CD37 is predominantly expressed on B cells with lower expression found in other immune cells [10]. CD53, on the other hand, was found to have a high and comparable expression over different immune cell types, possibly indicating a more general function within the immune system. Furthermore, CD37 has been found to possess ITIM-like and ITAM-like domains which are proposed to allow for direct signaling by CD37, while this is not the case for CD53 [11]. In terms of partner protein interactions, CD37 and CD53 have a few common partners such as β1 integrins and major histocompatibility complex proteins (MHC), whereas others are tetraspanin-specific, for example the interaction between PKC and CD53 or between suppressor of cytokine signaling 3 (SOCS3) and CD37 [12,13,14].
CD37 has been extensively studied and its loss has been linked to defects in numerous cellular and humoral immune functions such as migration, adhesion, proliferation, and antibody production [15,16,17,18]. The absence of CD37 results in spontaneous development of B cell lymphoma in mice and has been shown to correlate with worse progression-free and overall survival in lymphoma patients [13, 19]. In addition, multiple tetraspanins including CD37 have been shown to play a role in T cell activation, co-stimulation, proliferation, and immunological synapse formation [20, 21].
In contrast, CD53, also known as OX44 or TSPAN25, has been comparatively understudied despite strong evidence that this tetraspanin has important and non-redundant functions within the immune system [22,23,24,25]. CD53 is expressed highly on B cells and myeloid cells, though T cells also express it at significant levels [10, 26]. While there are many unanswered questions with respect to CD53, there are some functions specifically related to cell adhesion and signaling that have been ascribed to this protein, particularly in B and T cells. An overview of the proposed interaction partners of CD53 is shown in Table 1.
The aim of this review is to present a framework for the unique function of CD53 within the immune system based on the published literature. I will discuss the regulatory role of CD53 in different cellular processes with a focus on immune cell adhesion and signaling.
The role of CD53 in immune cell adhesion and migration
The ability of cells to adhere to one another and to the extracellular matrix (ECM) is important for maintaining the architecture and integrity of tissues and enabling cells to create the forces required for movement. Cell adhesion depends on cell adhesion molecules (CAMs) expressed on the cell surface, which include integrins, selectins, and cadherins. Cell adhesion plays an especially important role in the immune system, where adhesion molecules are used to stabilize immunological synapses between immune cells and facilitate their migration to sites of inflammation or infection [39, 40].
Many tetraspanins have been proposed to interact with CAMs, of which integrins in particular have been found to be key interaction partners [8, 41]. Integrins are heterodimeric adhesion molecules composed of an alpha and a beta subunit. They are involved primarily in cell–cell interactions and interactions between cells and the extracellular matrix (ECM). Tetraspanins have been reported to regulate integrin function through modulation of integrin signaling, by changing the distribution of integrins on the cell surface or by directing the trafficking of integrins [15, 42] For example, in T cells tetraspanins, CD9 and CD151 were found to accumulate at the immunological synapse. In the absence of these two tetraspanins integrin VLA-4 (α4β1) failed to accumulate at the cell–cell contact site which resulted in reduced T cell activation [20].
CD53 has also been linked to integrins and implicated in the regulation of adhesion of multiple immune cell types. Traditionally, the role of CD53 in adhesion has been studied using antibody ligation of CD53. For example, Todros-Dawda et al. showed that ligation of CD53 induced activation of the β2 integrin LFA-1 (αLβ2) in natural killer (NK) cells resulting in homotypic adhesion [22]. Furthermore, they proposed that CD53 ligation shifts NK cells towards a more proliferative phenotype, based on observed reductions in the degranulation responses of NK cells towards tumor cells, and impaired cytokine production.
The interaction between CD53 and LFA-1 is not restricted to NK cells as similar results have also been obtained in other immune cell types. In B and T cell lines the ligation of CD53, either via whole antibodies or via Fab’ fragments, also resulted in homotypic cell adhesion, which could be prevented through pre-treatment with antibodies against LFA-1 or its ligand ICAM-1 [27]. Based on these findings, the authors proposed that ligation of CD53 may affect cell activation resulting in increased cell adhesion which facilitates leukocyte adherence, extravasation, and aggregation to endothelia at sites of inflammation.
These findings were contradicted by the work of Lazo et al. who found that blocking of LFA-1 did not relieve homotypic adhesion of rat B cells induced by CD53 ligation [31]. Suggesting that this process may involve other adhesion molecules instead of, or in addition to, LFA-1. Supporting the involvement of other adhesion molecules, the authors found that the homotypic B cell adhesion could be prevented through addition of the chelating agents EDTA and EGTA, which remove the divalent cations required for integrin function. An alternative integrin to LFA-1 that may be involved is VLA-4, which has also been linked to CD53 through co-immunoprecipitation experiments, though the functional consequences of this interaction have remained unstudied [28]. Lazo et al. did not comment on the possible involvement of other integrins. Instead the authors suggested based on the application of inhibitors that signaling through tyrosine kinases and PKC might be responsible for the increased adhesive capacity of these cells. Moreover, the homotypic adhesion of these rat B cells was also completely abolished by inhibition of phosphoinositide 3-OH kinase (PI3K). The authors further supported this hypothesis by showing that de novo protein synthesis was a requisite for the homotypic adhesion induced by CD53 ligation, suggesting the involvement of intracellular signaling pathways.
While the abovementioned studies have made a good case for the involvement of CD53 in immune cell adhesion, they all suffered from the same shortcoming, namely, the exclusive use of antibodies to study CD53 function. While this method has been useful to identify potential partners of CD53, the results should be interpreted with caution as Fc receptor cross-linking may also play a role in the observed experimental outcomes. Modulation of the expression on CD53 through knockdowns, knockouts or overexpression are all viable alternatives to this method, as is illustrated by the following study.
Using an elegant Cd53−/− mouse model, Demaria et al. have shown that the homing of Cd53−/− lymphocytes to the lymph node is defective, with B cells more severely affected than T cells [25]. This was due to a severe decrease in the expression of the lymph node homing receptor L-selectin in Cd53−/− B cells. The impaired L-selectin expression in the absence of CD53 was confirmed in two human CD53−/− B cell lines. Further experiments revealed that the phenotype could be partially rescued by the application of a metalloprotease inhibitor suggesting that metalloproteases are partly responsible for the decrease in L-selectin expression when CD53 is absent. This was confirmed in experiments showing that CD53 inhibits the L-selectin shedding via ADAM17-dependent and -independent mechanisms. Finally, the authors showed that this defect in lymph node homing caused by the absence of CD53 resulted in a delayed adaptive immune response upon in vivo challenge with model antigens, illustrating the importance of this tetraspanin in the adaptive immune response.
Taken together the work discussed here illustrates the vital and varied role CD53 plays in regulating immune cell adhesion and migration, controlling this process through multiple distinctive adhesion molecules in different cell types. In addition, the work of Demaria et al. specifically, highlights the negative consequences associated with the absence of CD53 which result in pronounced immune dysfunction associated with an adhesion-related migration defect.
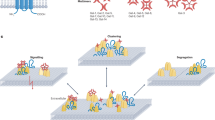
An overview of the most prominent interactions discussed in this section can be found in Fig. 1.
CD53 as a regulator of immune cell signaling
A healthy immune system depends on the intricate and reciprocal relationship that exists between immune cells and their environment. Central to this cellular interplay is the process of signaling, which involves receiving, transducing and responding to signals provided by neighboring cells or by the external surroundings. As the boundary between the intracellular and extracellular environment, the plasma membrane is vital to this process, providing a dynamic platform through which receptors activate signaling pathways.
Though signaling is a ubiquitous cell function, it has a particularly important role within the immune system which is dependent on signaling to recognize and eliminate pathogens and transformed cells. Tetraspanins help to support this function by forming TEMs that can function as platforms to support intense signaling activity. Different interactions between tetraspanins and signaling proteins have been reported, including both kinases and phosphatases [43].
CD53 has been found to interact with numerous membrane proteins involved in signaling. For example, CD2, which is expressed on the surface of NK and T cells, plays a role in cell adhesion and functions as a co-stimulatory molecule [44]. CD2 was identified as a partner of CD53 based on a screening of antibodies found to activate the phosphatidylinositol signaling pathway in a rat leukemia cell line with NK activity (RNK-16) [36]. Multiple antibodies identified in this screening were targeted to CD53, and were shown to elicit tyrosine phosphorylation, generate inositol phosphates and increase mobilization of calcium to the cytoplasm. Further investigation revealed that CD53 co-immunoprecipitated with CD2 in both NK and primary rat T lymphocytes and that stimulation via CD53 augmented TCR-mediated proliferation. Whether CD53 plays a role in the in vivo physiological activation of NK and T cells and whether this involves association with CD2 still remains unknown.
More recently a role for CD53 has been recognized as a regulator of B cell development via interleukin 7 receptor (IL-7R) signaling [37]. The authors identified a direct and specific interaction between CD53 and IL-7R through co-immunoprecipitation and proximity ligation assays. Subsequent experiments showed that B cells of mice lacking CD53 had a reduced surface expression of IL-7R accompanied by a significant decrease in bone marrow, splenic, lymphatic and peripheral B cells. These B cells also exhibited diminished PI3K and JAK/STAT signaling in the pro-B and pre-B cell developmental stage. The decrease in IL-7R expression is of interest because signaling via this receptor is required for early B cell survival and transition from pro-B to pre-B cell. In line with this, the authors reported increased cell death in developing Cd53−/− B cells and an associated reduction in pre-B and immature B cell populations. Not only does this study serve to further cement the role of CD53 in signaling, it also supports the premise that the immune system is reliant on CD53 in important and non-redundant ways.
Finally, numerous tetraspanins, including CD53, have been reported to associate with MHC class I (MHC-I) and class II (MHC-II) proteins [14]. CD53 was found to be part of a large complex on the surface of B cells which consisted of multiple tetraspanins, CD20 and MHC-I and -II. The authors speculated that these supramolecular complexes might play a role in signaling through MHC molecules and in antigen presentation to T cells, though they provided no evidence to support either claim. This proposed association is supported by findings from our own group using both immunoprecipitation and super-resolution microscopy which showed that CD53 could be immunoprecipitated with MHC-II and that CD53 was closer in proximity to MHC-II on the cell surface than other tetraspanins, indicating an association between these proteins [35]. In general, research into the relationship between tetraspanins and MHC molecules remains somewhat controversial due to conflicting results and the use of an antibody to identify tetraspanin-MHC interactions which later proved to be unspecific [45, 46]. Further research is required to concretely prove whether CD53 truly interacts with MHC molecules and what the functional consequences of this interaction may be.
Beyond membrane protein interactions, tetraspanins also have the ability to interact directly with intracellular signaling molecules, forming membrane hotspots for intense signaling activity. The foremost example of this is the interaction between tetraspanins and protein kinase C (PKC), which is especially relevant to tetraspanin CD53. In jurkat T cells PKCα has been found to co-immunoprecipitate reciprocally with numerous tetraspanins [29]. The authors observed that several tetraspanins, including CD53, created complexes that could link PKCs and integrins to each other to facilitate the PKC-dependent phosphorylation of integrins. This was supported by the finding that only integrins capable of interacting strongly with tetraspanins were found to interact with PKC. Further strengthening these finding is the work of Boscá et al. who showed that in rat macrophages the ligation of CD53 resulted in the mobilization of intracellular calcium and subsequent activation of PKC [30]. Similar CD53-mediated calcium fluxes have been observed in B cells, monocytes and granulocytes further substantiating this observation [47]. More recently our group has confirmed the existence of a direct interaction between CD53 and PKC in living immune cells [24]. In B cells we have shown that in the absence of CD53 the recruitment of PKCβ to the plasma membrane is impaired upon B cell receptor stimulation. This has implications for the activation of B cells, with CD53-negative primary B cells exhibiting impaired phosphorylation of PKC substrates. We were able to show that this is a result of a direct interaction between CD53 and PKCβ, as the deletion of a portion of the N-terminus of CD53 abrogates this interaction.
PKC is not the only kinase CD53 has been linked to. In B and T lymphoma cell lines the use of antibodies to ligate CD53 resulted in increased protein kinase B (Akt) phosphorylation which reduced the number of cells entering apoptosis upon serum deprivation [32]. Unfortunately, the intermediary between CD53 and the Akt pathway, whether this is a membrane or cytosolic protein, remains unknown. The authors proposed that this is a survival mechanism employed by tumor cells in poorly vascularized areas where nutrients and oxygen are scarce. The role of Akt in survival signaling is linked to the upstream kinase PI3K, which has been implicated in signaling related to CD53 as discussed above [31].
Like Akt, c-Jun N-terminal kinase (JNK) has also been identified as an effector protein functioning downstream of CD53 [33]. The antibody ligation of CD53 in both B and T cell lines was found to result in a fourfold increase in JNK activation which was transient and peaked at 3–5 min. In addition, the authors showed similar JNK activation in cells that normally do not express CD53 (renal cells, fibroblast) upon overexpression of CD53, suggesting that the role of CD53 in JNK activation is cell type independent. As with Akt, the mediator connecting CD53 to the JNK pathway is yet to be identified.
Kinases are not the only signaling molecules associated with CD53, almost three decades ago Carmo et al. provided initial evidence of an interaction between CD53 and an unidentified tyrosine phosphatase in lymph node cells and in a thymoma cell line [34]. This was based on immunoprecipitation experiments in which CD53-containing immune complexes were pulled down followed by kinase assays in the presence or absence of phosphatase inhibitors. These studies revealed that CD53 was associated with a tyrosine phosphatase capable of dephosphorylating Lck. The authors went on to present evidence that this tyrosine phosphatase was likely not CD45, but no further indication was provided as to the specific identity of this tyrosine phosphatase. Despite remaining unidentified, these results demonstrate that CD53 has a versatile function regulating both kinases and phosphatases and thereby contributing to the overall equilibrium of phosphorylation driving immune cell activation.
An overview of the most important interactions discussed in this section can be found in Fig. 1.
Outlook
Despite the fact that we have known for over 25 years that CD53 plays an important and non-redundant role in the immune system, it is only recently that we have begun to unravel the actual function of this tetraspanin [23]. Here, I have summarized the relevant literature regarding CD53 and its function within the immune system as a regulator of immune cell adhesion and signaling. Based on the data discussed in this review, I posit that CD53 has proven itself to be a versatile and important molecule in immune function worthy of further investigation.
The literature examined in this review gives rise to a number of unresolved subjects and questions I will discuss here. For example, CD53 is expressed on all cells of the lymphoid-myeloid lineage, yet the majority of research has been focused exclusively on B and T cell lines (Table 1). It would be of great benefit to explore the role of CD53 in other immune cells (such as antigen-presenting cells) and to further extend findings to primary cells and in vivo models.
Furthermore, the greater majority of research described here has relied on the use of antibody ligation to study CD53 function. Though this has no doubt been incredibly valuable in the past, we would be remiss not to take more advantage of the opportunities presented by novel techniques like CRISPR/Cas9 and the tetraspanin knockout mice established in the recent past to investigate CD53 function further. These tools allow for much more flexibility than the traditional antibody-based method and would permit us to begin designing studies to build on the fundamental knowledge we have gained in the last years.
In line with this, a strong case has been made for the interaction between CD53 and PKCs, which presents a unique opportunity to target this ubiquitous signaling molecule through an accessible surface protein. For many years, PKC inhibitors have been a target for intense research as an anti-cancer therapy [48]. These efforts have been hampered by the broad expression pattern of PKCs resulting in undesired adverse effects [49,50,51]. Based on this, it would be worthwhile to investigate the possibility of targeting PKC via CD53 in hematological malignancies, which provides not only an easily accessible membrane target but also an increased degree of specificity. Moreover, research has shown that the expression of CD53 is very high in radioresistant tumor cells, making this an even more attractive prospect as it may provide a new option in difficult to treat malignancies [52].
Beyond the matters raised in the literature discussed here, there are also many fundamental open questions related to CD53 which have not been addressed. For example, the exact composition of CD53 TEMs is still largely undefined. Which other partner proteins and lipids are contained by these microdomains and how do these contribute to CD53 function? Specifically, in the case of signaling there are numerous downstream pathways that have been linked to CD53, but the partner molecules mediating these effects have remained undefined.
Likewise, it would be interesting to further study what happens during activation of immune cells when CD53 is absent or impaired in its function. This is particularly interesting because cell activation is dependent on both the adhesive/migratory capacity of cells as well as their ability to signal, both of which have been linked to CD53 function. How CD53 would go about offsetting these functions in such a setting is therefore very interesting, particularly from the point of view of spatiotemporal dynamics. Finally, since CD53 has been proposed to interact with both kinases and phosphatases, it would be interesting to investigate how CD53 contributes to balancing signaling responses and whether this is dependent on factors such as timing, signal intensity, etc.
In conclusion, it is time to embrace a novel view of the immune system which recognizes the importance of membrane organizing proteins like CD53 and the central roles they can play in immunological processes. As a consequence, questions concerning how these organizing proteins function become increasingly important to our understanding of pathologies and the development of novel therapeutic strategies.
References
Min G, Wang H, Sun TT, Kong XP (2006) Structural basis for tetraspanin functions as revealed by the cryo-EM structure of uroplakin complexes at 6-Å resolution. J Cell Biol 173:975–983. https://doi.org/10.1083/jcb.200602086
Stipp CS, Kolesnikova TV, Hemler ME (2003) Functional domains in tetraspanin proteins. Trends Biochem Sci 28:106–112. https://doi.org/10.1016/S0968-0004(02)00014-2
Rubinstein E, Le Naour F, Lagaudrière-Gesbert C, Billard M, Conjeaud H, Boucheix C (1996) CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur J Immunol 26:2657–2665. https://doi.org/10.1002/eji.1830261117
Yáñez-Mó M, Barreiro O, Gordon-Alonso M, Sala-Valdés M, Sánchez-Madrid F (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19:434–446. https://doi.org/10.1016/j.tcb.2009.06.004
Jégou A, Ziyyat A, Barraud-Lange V, Perez E, Wolf JP, Pincet F, Gourier C (2011) CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc Natl Acad Sci USA 108:10946–10951. https://doi.org/10.1073/pnas.1017400108
Termini CM, Lidke KA, Gillette JM (2016) Tetraspanin CD82 regulates the spatiotemporal dynamics of PKCα in acute myeloid leukemia. Sci Rep 6:1–14. https://doi.org/10.1038/srep29859
Peñas PF, García-Diíez A, Saánchez-Madrid F, Yáñez-Mó M (2000) Tetraspanins are localized at motility-related structures and involved in normal human keratinocyte wound healing migration. J Invest Dermatol 114:1126–1135. https://doi.org/10.1046/j.1523-1747.2000.00998.x
Berditchevski F, Odintsova E (1999) Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol 146:477–492. https://doi.org/10.1083/jcb.146.2.477
Untemaehrer JJ, Chow A, Pypaert M, Inaba K, Mellman I (2007) The tetraspanin CD9 mediates lateral association of MHC class II molecules on the dendritic cell surface. Proc Natl Acad Sci USA 104:234–239. https://doi.org/10.1073/pnas.0609665104
de Winde CM, Zuidscherwoude M, Vasaturo A, van der Schaaf A, Figdor CG, van Spriel AB (2015) Multispectral imaging reveals the tissue distribution of tetraspanins in human lymphoid organs. Histochem Cell Biol 144:133–146. https://doi.org/10.1007/s00418-015-1326-2
Lapalombella R, Yeh Y-Y, Wang L, Ramanunni A, Rafiq S, Jha S, Staubli J, Lucas DM, Mani R, Herman SEM, Johnson AJ, Lozanski A, Andritsos L, Jones J, Flynn JM, Lannutti B, Thompson P, Algate P, Stromatt S, Jarjoura D, Mo X, Wang D, Chen C-S, Lozanski G, Heerema NA, Tridandapani S, Freitas MA, Muthusamy N, Byrd JC (2012) Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell 21:694–708. https://doi.org/10.1016/j.ccr.2012.03.040
Zuidscherwoude M, Dunlock V-ME, van den Bogaart G, van Deventer SJ, van der Schaaf A, van Oostrum J, Goedhart J, In’t Hout J, Hämmerling GJ, Tanaka S, Nadler A, Schultz C, Wright MD, Adjobo-Hermans MJW, van Spriel AB (2017) Tetraspanin microdomains control localized protein kinase C signaling in B cells. Sci Signal 10:2755. https://doi.org/10.1126/scisignal.aag2755
De Winde CM, Veenbergen S, Young KH, Xu-Monette ZY, Wang XX, Xia Y, Jabbar KJ, Van Den Brand M, Van Der Schaaf A, Elfrink S, Van Houdt IS, Gijbels MJ, Van De Loo FAJ, Bennink MB, Hebeda KM, Groenen PJTA, Van Krieken JH, Figdor CG, Van Spriel AB (2016) Tetraspanin CD37 protects against the development of B cell lymphoma. J Clin Invest 126:653–666. https://doi.org/10.1172/JCI81041
Szöllósi J, Horejsí V, Bene L, Angelisová P, Damjanovich S (1996) Supramolecular complexes of MHC class I, MHC class II, CD20, and tetraspan molecules (CD53, CD81, and CD82) at the surface of a B cell line JY. J Immunol 157:2939–2946
Wee JL, Schulze KE, Jones EL, Yeung L, Cheng Q, Pereira CF, Costin A, Ramm G, van Spriel AB, Hickey MJ, Wright MD (2015) Tetraspanin CD37 Regulates β 2 Integrin-Mediated Adhesion and Migration in Neutrophils. J Immunol 195:5770–5779. https://doi.org/10.4049/jimmunol.1402414
Gartlan KH, Wee JL, Demaria MC, Nastovska R, Chang TM, Jones EL, Apostolopoulos V, Pietersz GA, Hickey MJ, van Spriel AB, Wright MD (2013) Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur J Immunol 43:1208–1219. https://doi.org/10.1002/eji.201242730
van Spriel AB, Puls KL, Sofi M, Pouniotis D, Hochrein H, Orinska Z, Knobeloch K-P, Plebanski M, Wright MD (2004) A regulatory role for CD37 in T cell proliferation. J Immunol 172:2953–2961. https://doi.org/10.4049/jimmunol.172.5.2953
Knobeloch K-P, Wright MD, Ochsenbein AF, Liesenfeld O, Lohler J, Zinkernagel RM, Horak I, Orinska Z (2000) Targeted inactivation of the tetraspanin CD37 impairs T-cell-dependent B-cell response under suboptimal costimulatory conditions. Mol Cell Biol 20:5363–5369. https://doi.org/10.1128/mcb.20.15.5363-5369.2000
Xu-Monette ZY, Li L, Byrd JC, Jabbar KJ, Manyam GC, De Winde CM, Van Den Brand M, Tzankov A, Visco C, Wang J, Dybkaer K, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WWL, Huh J, Ponzoni M, Ferreri AJM, Møller MB, Parsons BM, Winter JN, Wang M, Hagemeister FB, Piris MA, Van Krieken JH, Medeiros LJ, Li Y, Van Spriel AB, Young KH (2016) Assessment of CD37 B-cell antigen and cell of origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood 128:3083–3100. https://doi.org/10.1182/blood-2016-05-715094
Rocha-Perugini V, González-Granado JM, Tejera E, López-Martín S, Yañez-Mó M, Sánchez-Madrid F (2014) Tetraspanins CD9 and CD151 at the immune synapse support T-cell integrin signaling. Eur J Immunol 44:1967–1975. https://doi.org/10.1002/eji.201344235
Tarrant JM, Groom J, Metcalf D, Li R, Borobokas B, Wright MD, Tarlinton D, Robb L (2002) The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro T-cell proliferative responses. Mol Cell Biol 22:5006–5018. https://doi.org/10.1128/mcb.22.14.5006-5018.2002
Todros-Dawda I, Kveberg L, Vaage JT, Inngjerdingen M (2014) The tetraspanin CD53 modulates responses from activating NK cell receptors, promoting LFA-1 activation and dampening NK cell effector functions. PLoS ONE. https://doi.org/10.1371/journal.pone.0097844
Mollinedo F, Fontan G, Barasoain I, Lazo PA (1997) Recurrent infectious diseases in human CD53 deficiency. Clin Diagn Lab Immunol 4:229–231. https://doi.org/10.1128/cdli.4.2.229-231.1997
Zuidscherwoude M, Dunlock V-ME, Van Den Bogaart G, Van Deventer SJ, Van Der Schaaf A, Van Oostrum J, Goedhart J, In’t Hout J, Hämmerling GJ, Tanaka S, Nadler A, Schultz C, Wright MD, Adjobo-Hermans MJW, Van Spriel AB (2017) Tetraspanin microdomains control localized protein kinase C signaling in B cells. Sci Signal. https://doi.org/10.1126/scisignal.aag2755
Demaria MC, Yeung L, Peeters R, Wee JL, Mihaljcic M, Jones EL, Nasa Z, Alderuccio F, Hall P, Smith BC, Binger KJ, Hammerling G, Kwok HF, Newman A, Ager A, van Spriel A, Hickey MJ, Wright MD (2020) Tetraspanin CD53 promotes lymphocyte recirculation by stabilising L-selectin surface expression. iScience. https://doi.org/10.1016/j.isci.2020.101104
Wright MD, Rochelle JM, Tomlinson MG, Seldln MF, Williams AF (1993) Gene structure, chromosomal localization, and protein sequence of mouse cd53 (Cd53): evidence that the transmembrane 4 superfamily arose by gene duplication. Int Immunol 5:209–216. https://doi.org/10.1093/intimm/5.2.209
Cao L, Yoshino T, Kawasaki N, Sakuma I, Takahashi K, Akagi T (1997) Anti-CD53 monoclonal antibody induced LFA-1/ICAM-1-dependent and -independent lymphocyte homotypic cell aggregation. Immunobiology 197:70–81. https://doi.org/10.1016/S0171-2985(97)80058-7
Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME (1996) Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29). J Immunol 157:2039–2047
Zhang XA, Bontrager AL, Hemler ME (2001) Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem 276:25005–25013. https://doi.org/10.1074/jbc.M102156200
Boscá L, Lazo PA (1994) Induction of nitric oxide release by MRC OX-44 (anti-CD53) through a protein kinase C-dependent pathway in rat macrophages. J Exp Med 179:1119–1126
Lazo PA, Cuevas L, Gutierrez del Arroyo A, Orúe E (1997) Ligation of CD53/OX44, a tetraspan antigen, induces homotypic adhesion mediated by specific cell-cell interactions. Cell Immunol 178:132–140. https://doi.org/10.1006/cimm.1997.1139
Yunta M, Lazo PA (2003) Apoptosis protection and survival signal by the CD53 tetraspanin antigen. Oncogene 22:1219–1224. https://doi.org/10.1038/sj.onc.1206183
Yunta M, Oliva JL, Barcia R, Horejsi V, Angelisova P, Lazo PA (2002) Transient activation of the c-Jun N-terminal kinase (JNK) activity by ligation of the tetraspan CD53 antigen in different cell types. Eur J Biochem 269:1012–1021. https://doi.org/10.1046/j.0014-2956.2001.02741.x
Carmo AM, Wright MD (1995) Association of the transmembrane 4 superfamily molecule CD53 with a tyrosine phosphatase activity. Eur J Immunol 25:2090–2095. https://doi.org/10.1002/eji.1830250743
Zuidscherwoude M, Göttfert F, Dunlock VME, Figdor CG, Van Den Bogaart G, Van Spriel AB (2015) The tetraspanin web revisited by super-resolution microscopy. Sci Rep. https://doi.org/10.1038/srep12201
Bell GM, Seaman WE, Niemi EC, Imboden JB (1992) The OX-44 molecule couples to signaling pathways and is associated with CD2 on rat T lymphocytes and a natural killer cell line. J Exp Med 175:527–536. https://doi.org/10.1084/jem.175.2.527
Greenberg ZJ, Monlish DA, Bartnett RL, Yang Y, Shen G, Li W, Bednarski JJ, Schuettpelz LG (2020) The tetraspanin CD53 regulates early B cell development by promoting IL-7R signaling. J Immunol 204:58–67. https://doi.org/10.4049/jimmunol.1900539
Nichols TC, Guthridge JM, Karp DR, Molina H, Fletcher DR, Holers VM (1998) γ-Glutamyl transpeptidase, an ecto-enzyme regulator of intracellular redox potential, is a component of TM4 signal transduction complexes. Eur J Immunol 28:4123–4129. https://doi.org/10.1002/(SICI)1521-4141(199812)28:12%3c4123:AID-IMMU4123%3e3.0.CO;2-G
Jankowska KI, Williamson EK, Roy NH, Blumenthal D, Chandra V, Baumgart T, Burkhardt JK (2018) Integrins modulate T cell receptor signaling by constraining actin flow at the immunological synapse. Front Immunol 9:1–19. https://doi.org/10.3389/fimmu.2018.00025
Mittelbrunn M, Molina A, Escribese MM, Yáñez-Mó M, Escudero E, Ursa Á, Tejedor R, Mampaso F, Sánchez-Madrid F (2004) VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci USA 101:11058–11063. https://doi.org/10.1073/pnas.0307927101
Bassani S, Cingolani LA (2012) Tetraspanins: interactions and interplay with integrins. Int J Biochem Cell Biol 44:703–708. https://doi.org/10.1016/j.biocel.2012.01.020
Reyes R, Monjas A, Yánez-Mó M, Cardeñes B, Morlino G, Gilsanz A, Machado-Pineda Y, Lafuente E, Monk P, Sánchez-Madrid F, Cabañas C (2015) Different states of integrin LFA-1 aggregation are controlled through its association with tetraspanin CD9. Biochim Biophys Acta Mol Cell Res 1853:2464–2480. https://doi.org/10.1016/j.bbamcr.2015.05.018
Levy S, Shoham T (2005) The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol 5:136–148. https://doi.org/10.1038/nri1548
Moingeon P, Chang H-C, Wallner BP, Stebbins C, Frey AZ, Reinherz EL (1989) CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature 339:312–314
Poloso NJ, Denzin LK, Roche PA (2006) CDw78 defines MHC class II-peptide complexes that require Ii Chain-dependent lysosomal trafficking, not localization to a specific tetraspanin membrane microdomain. J Immunol 177:5451–5458. https://doi.org/10.4049/jimmunol.177.8.5451
Kropshofer H, Spindeldreher S, Röhn TA, Platania N, Grygar C, Daniel N, Wölpl A, Langen H, Horejsi V, Vogt AB (2002) Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat Immunol 3:61–68. https://doi.org/10.1038/ni750
Olweus J, Lund-Johansen F, Horejsi V (1993) CD53, a protein with four membrane-spanning domains, mediates signal transduction in human monocytes and B cells. J Immunol 151:707–716
Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG (2014) Protein kinase C and cancer: what we know and what we do not. Oncogene 33:5225–5237. https://doi.org/10.1038/onc.2013.524
Robertson MJ, Kahl BS, Vose JM, de Vos S, Laughlin M, Flynn PJ, Rowland K, Cruz JC, Goldberg SL, Musib L, Darstein C, Enas N, Kutok JL, Aster JC, Neuberg D, Savage KJ, LaCasce A, Thornton D, Slapak CA, Shipp MA (2007) Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 25:1741–1746. https://doi.org/10.1200/JCO.2006.09.3146
Cooke M, Magimaidas A, Casado-Medrano V, Kazanietz MG (2017) Protein kinase C in cancer: the top five unanswered questions. Mol Carcinog 56:1531–1542. https://doi.org/10.1002/mc.22617
Mochly-Rosen D, Das K, Grimes KV (2012) Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11:937–957. https://doi.org/10.1038/nrd3871
Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, Herzenberg LA, Steinman L, Herzenberg LA (2000) Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci USA 97:2680–2685. https://doi.org/10.1073/pnas.97.6.2680
Acknowledgements
I acknowledge funding support from the European Research Council (Consolidator Grant 724281 to A. B. van Spriel) and thank Prof. A. B. van Spriel and Dr. N. H. Revelo for their critical evaluation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Edited by Luise Florin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Special Issue on Tetraspanins in Infection and Immunity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dunlock, V.E. Tetraspanin CD53: an overlooked regulator of immune cell function. Med Microbiol Immunol 209, 545–552 (2020). https://doi.org/10.1007/s00430-020-00677-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-020-00677-z