Abstract
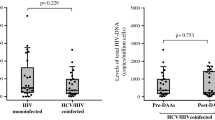
This longitudinal study described cellular HIV-DNA changes and their correlation with HIV low-level plasma viremia (LLV) in HIV–HCV co-infected patients on successful antiretroviral and anti-HCV therapy by treatment with direct-acting antivirals (DAA). Thirty-nine patients were examined prior to the start of DAA (T0), after week 12 (T1) and 24 weeks (T2) of anti-HCV therapy. Cellular PBMC HIV-DNA was analysed as an absolute value and as the percentage of increase or decrease from T0 to T2. Patients were classified as having undetectable plasma HIV viraemia (UV) or LLV in the year before the start of anti-HCV treatment and within the T0–T2 study period. Thirty-five patients (89.7%) of the 39 subjects enrolled had the same plasma HIV viraemia control in the year before HCV treatment and in the T0–T2 interval. The HIV-DNA value at T0 and at T2 was higher in patients with LLV than in subjects with UV (p = 0.015 and p = 0.014, respectively). A similar proportion of patients with LLV and UV experienced an increase or decrease of HIV-DNA from T0 to T2. The percentage increase in HIV-DNA value (262.8%) from T0 to T2 was higher compared to the decrease (43.5%) in patients with UV (p = 0.012), and it was higher compared to the percentage increase in HIV-DNA value reported in subjects with LLV (262.8 versus 49%, p = 0.026). HIV–HCV co-infected patients experienced a multifaceted perturbation of cellular HIV-DNA levels within a 24-week period during anti-HCV treatment; the extent of the phenomenon was greater in subjects with UV. Fast HCV-RNA clearance seemed to have a greater influence on the cellular reservoir than on plasma HIV-RNA.



Similar content being viewed by others
References
Mandorfer M, Schwabl P, Steiner S, Reiberger T, Peck-Radosavljevic M (2016) Advances in the management of HIV/HCV coinfection. Hepatol Int 10:424–435. doi:10.1007/s12072-015-9691-4
Günthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, Hoy JF, Mugavero MJ, Sax PE, Thompson MA, Gandhi RT, Landovitz RJ, Smith DM, Jacobsen DM, Volberding PA (2016) Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA 316:191–210. doi:10.1001/jama.2016.8900
European AIDS Clinical Society Guidelines, Version 8.0—June 2016. http://www.eacsociety.org. Accessed 18 July 2016
Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, Chen P, Chen BK, Klotman ME, Bansal MB (2010) Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 52:612–622. doi:10.1002/hep.23679
Shmagel KV, Saidakova EV, Shmagel NG, Korolevskaya LB, Chereshnev VA, Robinson J, Grivel JC, Douek DC, Margolis L, Anthony DD, Lederman MM (2016) Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med 17:581–589. doi:10.1111/hiv.12357
Sumpter R Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M Jr (2005) Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol 79:2689–2699. doi:10.1128/JVI.79.5.2689-2699.2005
Mavigner M, Delobel P, Cazabat M, Dubois M, L’faqihi-Olive FE, Raymond S, Pasquier C, Marchou B, Massip P, Izopet J (2009) HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One 4:e7658. doi:10.1371/journal.pone.0007658
Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H (2008) Residual viraemia in HIV-1-infected patients with plasma viral load <20 copies/mL is associated with increased blood levels of soluble immune activation markers. Scand J Immunol 68:652–660
Baroncelli S, Pirillo MF, Galluzzo CM, Antoni AD, Ladisa N, Francisci D, d’Ettorre G, Segala D, Vivarelli A, Sozio F, Cirioni O, Weimer LE, Fragola V, Parruti G, Floridia M (2015) Rate and determinants of residual viremia in multidrug-experienced patients successfully treated with raltegravir-based regimens. AIDS Res Hum Retrovir 31:71–77. doi:10.1089/AID.2014.0060
Pugliese P, Delpierre C, Cuzin L, Poizot-Martin I, Rey D, Saune K, Cottalorda J, Bettinger D, Delaugerre C, Hoen B, Dat AIDS Study Group (2013) An undetectable polymerase chain reaction signal in routine HIV plasma viral load monitoring is associated with better virological outcomes in patients receiving highly active antiretroviral therapy. HIV Med 14:509–515. doi:10.1111/hiv.12041
Calcagno A, Motta I, Ghisetti V, Lo Re S, Allice T, Marinaro L, Milia MG, Tettoni MC, Trentini L, Orofino G, Salassa B, Di Perri G, Bonora S (2015) HIV-1 very low level viremia is associated with virological failure in highly active antiretroviral treatment-treated patients. AIDS Res Hum Retrovir 31:999–1008. doi:10.1089/AID.2015.0102
Cho H, Kikuchi M, Li Y, Nakamoto N, Amorosa VK, Valiga ME, Chang KM (2014) Induction of multiple immune regulatory pathways with differential impact in HCV/HIV coinfection. Front Immunol 5:265. doi:10.3389/fimmu.2014.00265
Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS (1998) Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA 95:8869–8873
Han Y, Wind-Rotolo M, Yang HC, Siliciano JD, Siliciano RF (2007) Experimental approaches to the study of HIV-1 latency. Nat Rev Microbiol 5:95–106. doi:10.1038/nrmicro1580
Fischer M, Joos B, Niederöst B, Kaiser P, Hafner R, von Wyl V, Ackermann M, Weber R, Günthard HF (2008) Biphasic decay kinetics suggest progressive slowing in turnover of latently HIV-1 infected cells during antiretroviral therapy. Retrovirology 5:107. doi:10.1186/1742-4690-5-107
Parisi SG, Andreis S, Mengoli C, Scaggiante R, Ferretto R, Manfrin V, Cruciani M, Giobbia M, Boldrin C, Basso M, Andreoni M, Palù G, Sarmati L (2012) Baseline cellular HIV DNA load predicts HIV DNA decline and residual HIV plasma levels during effective antiretroviral therapy. J Clin Microbiol 50:258–263. doi:10.1128/JCM.06022-11
Parisi SG, Sarmati L, Andreis S, Scaggiante R, Cruciani M, Ferretto R, Manfrin V, Basso M, Andreoni M, Mengoli C, Palù G (2015) Strong and persistent correlation between baseline and follow-up HIV-DNA levels and residual viremia in a population of naïve patients with more than 4 years of effective antiretroviral therapy. Clin Microbiol Infect 21:288.e5–288.e7. doi:10.1016/j.cmi.2014.10.009
Kiselinova M, De Spiegelaere W, Buzon MJ, Malatinkova E, Lichterfeld M, Vandekerckhove L (2016) Integrated and total HIV-1 DNA predict ex vivo viral outgrowth. PLoS Pathog 12:e1005472. doi:10.1371/journal.ppat.1005472
Wong JK, Günthard HF, Havlir DV, Zhang ZQ, Haase AT, Ignacio CC, Kwok S, Emini E, Richman DD (1997) Reduction of HIV-1-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci 94:12574–12579
Sarmati L, Parisi SG, Nicastri E, d’Ettorre G, Andreoni C, Dori L, Gatti F, Montano M, Buonomini AR, Boldrin C, Palù G, Vullo V, Andreoni M (2007) Cellular HIV-1 DNA quantitation in patients during simplification therapy with protease inhibitor-sparing regimens. J Med Virol 79:880–886. doi:10.1002/jmv.20914
Sarmati L, Parisi SG, Nicastri E, d’Ettorre G, Palmisano L, Andreotti M, Andreoni C, Giuliano M, Gatti F, Boldrin C, Palù G, Vullo V, Vella S, Andreoni M (2005) Association between cellular human immunodeficiency virus DNA level and immunological parameters in patients with undetectable plasma viremia level during highly active antiretroviral therapy. J Clin Microbiol 43:6183–6185. doi:10.1128/JCM.43.12.6183-6185.2005
Widera M, Dirks M, Bleekmann B, Jablonka R, Däumer M, Walter H, Ehret R, Verheyen J, Esser S (2017) HIV-1 persistent viremia is frequently followed by episodes of low-level viremia. Med Microbiol Immunol 206:203–215
Morón-López S, Gómez-Mora E, Salgado M, Ouchi D, Puertas MC, Urrea V, Navarro J, Jou A, Pérez M, Tural C, Clotet B, Montaner LJ, Blanco J, Crespo M, Martinez-Picado J (2016) Short-term treatment with interferon alfa diminishes expression of HIV-1 and reduces CD4 + T-cell activation in patients coinfected with HIV and hepatitis C Virus and receiving antiretroviral therapy. J Infect Dis 213:1008–1012. doi:10.1093/infdis/jiv521
Jiao YM, Weng WJ, Gao QS, Zhu WJ, Cai WP, Li LH, Li HJ, Gao YQ, Wu H (2015) Hepatitis C therapy with interferon-α and ribavirin reduces the CD4 cell count and the total, 2LTR circular and integrated HIV-1 DNA in HIV/HCV co-infected patients. Antivir Res 118:118–122. doi:10.1016/j.antiviral.2015.03.011
Sun H, Buzon MJ, Shaw A, Berg RK, Yu XG, Ferrando-Martinez S, Leal M, Ruiz-Mateos E, Lichterfeld M (2014) Hepatitis C therapy with interferon-α and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis 209:1315–1320. doi:10.1093/infdis/jit628
Piroth L, Wittkop L, Lacombe K, Rosenthal E, Gilbert C, Miailhes P, Carrieri P, Chas J, Poizot-Martin I, Gervais A, Dominguez S, Neau D, Zucman D, Billaud E, Morlat P, Aumaitre H, Lascoux-Combe C, Simon A, Bouchaud O, Teicher E, Bani-Sadr F, Alric L, Vittecoq D, Boué F, Duvivier C, Valantin MA, Esterle L, Dabis F, Sogni P, Salmon D, ANRS CO13 HEPAVIH study group (2017) Efficacy and safety of direct-acting antiviral regimens in HIV/HCV-co-infected patients—French ANRS CO13 HEPAVIH cohort. J Hepatol 67:23–31. doi:10.1016/j.jhep.2017.02.012
Sarmati L, D’Ettorre G, Parisi SG, Andreoni M (2015) HIV replication at low copy number and its correlation with the HIV reservoir: a clinical perspective. Curr HIV Res 13:250–257. doi:10.2174/1570162X13666150407142539
Sánchez-Conde M, Montes-Ramírez ML, Miralles P, Alvarez JM, Bellón JM, Ramírez M, Arribas JR, Gutiérrez I, López JC, Cosín J, Alvarez E, González J, Berenguer J (2010) Comparison of transient elastography and liver biopsy for the assessment of liver fibrosis in HIV/hepatitis C virus-coinfected patients and correlation with noninvasive serum markers. J Viral Hepat 17:280–286. doi:10.1111/j.1365-2893.2009.01180
Hepatitis Drug Interactions website, website, http://www.hep-druginteractions.org. Accessed 19 July 2016
Gianella S, Anderson CM, Vargas MV, Richman DD, Little SJ, Morris SR, Smith DM (2013) Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis 207:898–902. doi:10.1093/infdis/jis777
Gianella S, Anderson CM, Var SR, Oliveira MF, Lada SM, Vargas MV, Massanella M, Little SJ, Richman DD, Strain MC, Pérez-Santiago J, Smith DM (2016) Replication of human herpes viruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol 90:3944–3952. doi:10.1128/JVI.02638-15
Debes JD, de Knegt RJ, Boonstra A (2017) The path to cancer and back: immune modulation during hepatitis C virus infection, progression to fibrosis and cancer, and unexpected roles of new antivirals. Transplantation 101:910–915. doi:10.1097/TP.0000000000001623
Langhans B, Nischalke HD, Krämer B, Hausen A, Dold L, van Heteren P, Hüneburg R, Nattermann J, Strassburg CP, Spengler U (2017) Increased peripheral CD4 + regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol 66:888–896. doi:10.1016/j.jhep.2016.12.019
Hengst J, Falk CS, Schlaphoff V, Deterding K, Manns MP, Cornberg M (2016) Wedemeyer H (2016) Direct-acting antiviral-induced hepatitis C virus clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. J Infect Dis 214:1965–1974
Hengst J, Strunz B, Deterding K, Ljunggren HG, Leeansyah E, Manns MP, Cornberg M, Sandberg JK, Wedemeyer H, Björkström NK (2016) Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur J Immunol 46:2204–2210. doi:10.1002/eji.201646447
Chen F, Zhang J, Wen B, Luo S, Lin Y, Ou W, Guo F, Tang P, Liu W, Qu X (2016) HBV/HCV dual infection impacts viral load, antibody response, and cytokine expression differently from HBV or HCV single infection. Sci Rep 6:39409. doi:10.1038/srep39409
Raimondo G, Brunetto MR, Pontisso P, Smedile A, Maina AM, Saitta C, Squadrito G, Tono N, Associazione Italiana Studio Fegato Cooperative Group (2006) Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology 43:100–107. doi:10.1002/hep.20944
Reuter S, Oette M, Wilhelm FC, Beggel B, Kaiser R, Balduin M, Schweitzer F, Verheyen J, Adams O, Lengauer T, Fätkenheuer G, Pfister H, Häussinger D (2011) Prevalence and characteristics of hepatitis B and C virus infections in treatment-naïve HIV-infected patients. Med Microbiol Immunol 200:39–49. doi:10.1007/s00430-010-0172-z
Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X, Henry AR, Laboune F, Hu J, Ambrozak D, Hughes MS, Hoh R, Casazza JP, Vostal A, Bunis D, Nganou-Makamdop K, Lee JS, Migueles SA, Koup RA, Connors M, Moir S, Schacker T, Maldarelli F, Hughes SH, Deeks SG, Douek DC (2016) Multiple origins of virus persistence during natural control of HIV infection. Cell 166:1004–1015. doi:10.1016/j.cell.2016.06.039
Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, Chung YS, Penugonda S, Chipman JG, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR, Wolinsky SM (2016) Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530:51–56. doi:10.1038/nature16933
Kondo Y, Ueno Y, Kakazu E, Kobayashi K, Shiina M, Tamai K, Machida K, Inoue J, Wakui Y, Fukushima K, Obara N, Kimura O, Shimosegawa T (2011) Lymphotropic HCV strain can infect human primary naïve CD4 + cells and affect their proliferation and IFN-γ secretion activity. J Gastroenterol 46:232–241. doi:10.1007/s00535-010-0297-2
Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, Porcella S, Wang H, Herrmann E, McHutchison J, Suffredini AF, Polis M, Hewitt S, Prokunina-Olsson L, Masur H, Fauci AS, Kottilil S (2014) Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Investig 124:3352–3363. doi:10.1172/JCI75938
Stephan C, Baldauf HM, Barry J, Giordano FA, Bartholomae CC, Haberl A, Bickel M, Schmidt M, Laufs S, Kaderali L, Keppler OT (2014) Impact of raltegravir on HIV-1 RNA and DNA forms following initiation of antiretroviral therapy in treatment-naive patients. J Antimicrob Chemother 69:2809–2818. doi:10.1093/jac/dku213
Koelsch KK, Boesecke C, McBride K, Gelgor L, Fahey P, Natarajan V, Baker D, Bloch M, Murray JM, Zaunders J, Emery S, Cooper DA, Kelleher AD, PINT study team (2011) Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS 25:2069–2078. doi:10.1097/QAD.0b013e32834b9658
Rossetti B, Meini G, Bianco C, Lamonica S, Mondi A, Belmonti S, Fanti I, Ciccarelli N, Di Giambenedetto S, Zazzi M, De Luca A (2017) Total cellular HIV-1 DNA decreases after switching to raltegravir-based regimens in patients with suppressed HIV-1 RNA. J Clin Virol 91:18–24. doi:10.1016/j.jcv.2017.03.018
Abad-Fernández M, Dronda F, Moreno A, Casado JL, Pérez-Elías MJ, Quereda C, Moreno S, Vallejo A (2015) Brief Report: Reduced cell-associated HTLV-2 DNA in antiretroviral treated HIV-1-HCV-coinfected patients who either received interferon-α/ribavirin-based hepatitis C therapy or had spontaneous HCV RNA clearance. J Acquir Immune Defic Syndr 69:286–290. doi:10.1097/QAI.0000000000000608
Turci M, Pilotti E, Ronzi P, Magnani G, Boschini A, Parisi SG, Zipeto D, Lisa A, Casoli C, Bertazzoni U (2006) Coinfection with HIV-1 and human T-Cell lymphotropic virus type II in intravenous drug users is associated with delayed progression to AIDS. J Acquir Immune Defic Syndr 41:100–106. doi:10.1097/01.qai.0000179426.04166.12
Massimo A, Teti E, Antinori A, Milazzoi L, Sollima S, Rizzardini G, Di Biagio A, Saracino A, Bruno R, Borghi V, De Luca A, Cattelan A, Hasson H, Taliani G, Monforte AD, Mastroianni CM, Di Perri G, Bigoni S, Puoti M, Spinetti A, Gori A, Boffa N, Bruno C, Giacometti A, Parruti G, Vullo V, Chirianni A, Pennica A, Pasquazzi C, Segala D, Sarmati L, SIMIT (Società Italiana di Malattie Infettive e Tropicali) (2017) Ombitasvir/paritaprevir/ritonavir and dasabuvir combination treatment in patients with HIV/HCV Co-Infection: results of an Italian compassionate use program. Clin Infect Dis 64:680–683. doi:10.1093/cid/ciw846
Bruno G, Saracino A, Fabrizio C, Scudeller L, Milano E, Dell’Acqua R, Ladisa N, Fasano M, Minniti S, Buccoliero G, Tartaglia A, Giammario A, Milella M, Angarano G (2017) Safety and effectiveness of a 12-week course of sofosbuvir and simeprevir ± ribavirin in HCV-infected patients with or without HIV infection: a multicentre observational study. Int J Antimicrob Agents 49:296–301. doi:10.1016/j.ijantimicag.2016.11.030
Lacombe K, Fontaine H, Dhiver C, Metivier S, Rosenthal E, Antonini T, Valantin MA, Miailhes P, Harent S, Batisse D, Pageaux GP, Chas J, Aumaitre H, Dominguez S, Allegre T, Lafeuillade A, Billaud E, De Truchis P, Perre P, Leroy V, De Ledinghen V, Sogni P, Dabis F, Zhao Y, Filipovics A, Fedchuk L, Akremi R, Bennai Y, Salmon Ceron D (2017) Real-world efficacy of daclatasvir and sofosbuvir, with and without ribavirin, in HIV/HCV coinfected patients with advanced liver disease in a French early access cohort. J Acquir Immune Defic Syndr 75:97–107. doi:10.1097/QAI.0000000000001342
Russelli G, Pizzillo P, Iannolo G, Barbera F, Tuzzolino F, Liotta R, Traina M, Vizzini G, Gridelli B, Badami E, Conaldi PG (2017) HCV replication in gastrointestinal mucosa: potential extra-hepatic viral reservoir and possible role in HCV infection recurrence after liver transplantation. PLoS One 12:e0181683. doi:10.1371/journal.pone.0181683
Tully DC, Hjerrild S, Leutscher PD, Renvillard SG, Ogilvie CB, Bean DJ, Videbech P, Allen TM, McKeating JA, Fletcher NF (2016) Deep sequencing of hepatitis C virus reveals genetic compartmentalization in cerebrospinal fluid from cognitively impaired patients. Liver Int 36:1418–1424. doi:10.1111/liv.13134
Svicher V, Ceccherini-Silberstein F, Antinori A, Aquaro S, Perno CF (2014) Understanding HIV compartments and reservoirs. Curr HIV/AIDS Rep 11:186–194. doi:10.1007/s11904-014-0207-y
Lambert-Niclot S, Flandre P, Valantin MA, Peytavin G, Duvivier C, Haim-Boukobza S, Algarte-Genin M, Yazdanpanah Y, Girard PM, Katlama C, Calvez V, Marcelin AG (2011) Factors associated with virological failure in HIV-1-infected patients receiving darunavir/ritonavir monotherapy. J Infect Dis 204:1211–1216. doi:10.1093/infdis/jir518
Donath M, Wolf T, Stürmer M, Herrmann E, Bickel M, Khaykin P, Göpel S, Gute P, Haberl A, de Leuw P, Schüttfort G, Berger A, Stephan C, for Frankfurt HIV Cohort Study (2016) HIV-1 replication in central nervous system increases over time on only protease inhibitor therapy. Med Microbiol Immunol 205:575–583
Acknowledgements
This work was supported by MURST ex 60% (60A07-2972/15) to MB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and local legislation and was approved by the local Ethics Committee (2606-12P).
Informed consent
The enrolled subjects provided informed consent for all procedures and the use of their blinded data for scientific evaluation and publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parisi, S.G., Andreis, S., Basso, M. et al. Time course of cellular HIV-DNA and low-level HIV viremia in HIV–HCV co-infected patients whose HCV infection had been successfully treated with directly acting antivirals. Med Microbiol Immunol 206, 419–428 (2017). https://doi.org/10.1007/s00430-017-0518-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-017-0518-x