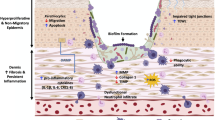
Abstract
Diabetic patients are more susceptible to the development of chronic wounds than non-diabetics. The impaired healing properties of these wounds, which often develop debilitating bacterial infections, significantly increase the rate of lower extremity amputation in diabetic patients. We hypothesize that bacterial biofilms, or sessile communities of bacteria that reside in a complex matrix of exopolymeric material, contribute to the severity of diabetic wounds. To test this hypothesis, we developed an in vivo chronic wound, diabetic mouse model to determine the ability of the opportunistic pathogen, Pseudomonas aeruginosa, to cause biofilm-associated infections. Utilizing this model, we observed that diabetic mice with P. aeruginosa-infected chronic wounds displayed impaired bacterial clearing and wound closure in comparison with their non-diabetic littermates. While treating diabetic mice with insulin improved their overall health, it did not restore their ability to resolve P. aeruginosa wound infections or speed healing. In fact, the prevalence of biofilms and the tolerance of P. aeruginosa to gentamicin treatment increased when diabetic mice were treated with insulin. Insulin treatment was observed to directly affect the ability of P. aeruginosa to form biofilms in vitro. These data demonstrate that the chronically wounded diabetic mouse appears to be a useful model to study wound healing and biofilm infection dynamics, and suggest that the diabetic wound environment may promote the formation of biofilms. Further, this model provides for the elucidation of mechanistic factors, such as the ability of insulin to influence antimicrobial effectiveness, which may be relevant to the formation of biofilms in diabetic wounds.





Similar content being viewed by others
References
Centers for Disease Control and Prevention (2011) National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. US Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta
Reiber GE (1996) The epidemiology of diabetic foot problems. Diabet Med 13(Suppl 1):S6–11
Ohsawa S, Inamori Y, Fukuda K, Hirotuji M (2001) Lower limb amputation for diabetic foot. Arch Orthop Trauma Surg 121(4):186–190
Ebskov B, Josephsen P (1980) Incidence of reamputation and death after gangrene of the lower extremity. Prosthet Orthot Int 4(2):77–80
Armstrong DG, Lavery LA, Harkless LB, Van Houtum WH (1997) Amputation and reamputation of the diabetic foot. J Am Podiatr Med Assoc 87(6):255–259
Cunningham AB (2006) Biofilms:The Hypertextbook. Montana State University, Bozeman
Yoga R, Khairul A, Sunita K, Suresh C (2006) Bacteriology of diabetic foot lesions. Med J Mala 61 Suppl A:14–16
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193
Ehrlich GD, Veeh R, Wang X, Costerton JW, Hayes JD, Hu FZ, Daigle BJ, Ehrlich MD, Post JC (2002) Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287(13):1710–1715
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP (2000) Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407(6805):762–764
Slusher MM, Myrvik QN, Lewis JC, Gristina AG (1987) Extended-wear lenses, biofilm, and bacterial adhesion. Arch Ophthalmol 105(1):110–115
Edwards R, Harding KG (2004) Bacteria and wound healing. Curr Opin Infect Dis 17(2):91–96
James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS (2008) Biofilms in chronic wounds. Wound Repair Regen 16(1):37–44
Wolcott RD, Ehrlich GD (2008) Biofilms and chronic infections. JAMA 299(22):2682–2684
Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, Hoiby N, Givskov M (2008) Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 16(1):2–10. doi:10.1111/j.1524-475X.2007.00283.x
Holder IA, Brown RL, Greenhalgh DG (1997) Mouse models to study wound closure and topical treatment of infected wounds in healing-impaired and normal healing hosts. Wound Repair Regen 5:198–204
Tesch GH, Allen TJ (2007) Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 12(3):261–266. doi:10.1111/j.1440-1797.2007.00796.x
Schaber JA, Triffo WJ, Suh SJ, Oliver JW, Hastert MC, Griswold JA, Auer M, Hamood AN, Rumbaugh KP (2007) Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect Immun 75(8):3715–3721
Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, Dowd SE (2010) Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care 19(8):320–328
Lyte M, Ernst S (1992) Catecholamine induced growth of gram negative bacteria. Life Sci 50(3):203–212
Hammond A, Dertien J, Colmer-Hamood JA, Griswold JA, Hamood AN (2010) Serum inhibits P. aeruginosa biofilm formation on plastic surfaces and intravenous catheters. J Surg Res 159(2):735–746. doi:10.1016/j.jss.2008.09.003
Shen X, Bornfeldt KE (2007) Mouse models for studies of cardiovascular complications of type 1 diabetes. Ann N Y Acad Sci 1103:202–217. doi:10.1196/annals.1394.004
Spanheimer RG, Umpierrez GE, Stumpf V (1988) Decreased collagen production in diabetic rats. Diabetes 37(4):371–376
Covington DS, Xue H, Pizzini R, Lally KP, Andrassy RJ (1993) Streptozotocin and alloxan are comparable agents in the diabetic model of impaired wound healing. Diabetes Res 23(2):47–53
Jt Michaels, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC (2007) db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen 15(5):665–670. doi:10.1111/j.1524-475X.2007.00273.x
Kania RE, Lamers GE, Vonk MJ, Huy PT, Hiemstra PS, Bloemberg GV, Grote JJ (2007) Demonstration of bacterial cells and glycocalyx in biofilms on human tonsils. Arch Otolaryngol Head Neck Surg 133(2):115–121. doi:10.1001/archotol.133.2.115
Akiyama H, Huh WK, Yamasaki O, Oono T, Iwatsuki K (2002) Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin? Br J Dermatol 147(5):879–885
Anderson GG, O’Toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol 322:85–105
Plotkin BJ, Viselli SM (2000) Effect of insulin on microbial growth. Curr Microbiol 41(1):60–64
Jeromson S, Keig P, Kerr K (1999) Interaction of insulin and Burkholderia cepacia. Clin Microbiol Infect 5(7):439–442
Hayford JT, Thompson RG (1982) Free and total insulin integrated concentrations in insulin dependent diabetes. Metabolism 31(4):387–397. doi:10026-0495(82)90116-0
Hirose H, Takayama T, Hozawa S, Hibi T, Saito I (2011) Prediction of metabolic syndrome using artificial neural network system based on clinical data including insulin resistance index and serum adiponectin. Comput Biol Med 41(11):1051–1056. doi:10.1016/j.compbiomed.2011.09.005
Yang M, Guo Q, Zhang X, Sun S, Wang Y, Zhao L, Hu E, Li C (2009) Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. Int J Nurs Stud 46(6):753–758. doi:10.1016/j.ijnurstu.2009.01.004
Woods DE, Jones AL, Hill PJ (1993) Interaction of insulin with Pseudomonas pseudomallei. Infect Immun 61(10):4045–4050
Singh VA, Barbul A (2008) Bacterial biofilms in wounds. Wound Repair Regen 16(1):1. doi:10.1111/j.1524-475X.2007.00349.x
Kanno E, Toriyabe S, Zhang L, Imai Y, Tachi M (2009) Biofilm formation on rat skin wounds by Pseudomonas aeruginosa carrying the green fluorescent protein gene. Exp Dermatol. doi:10.1111/j.1600-0625.2009.00931.x
Schierle CF, De la Garza M, Mustoe TA, Galiano RD (2009) Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 17(3):354–359. doi:10.1111/j.1524-475X.2009.00489.x
Seth AK, Geringer MR, Gurjala AN, Hong SJ, Galiano RD, Leung KP, Mustoe TA (2012) Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plast Reconstr Surg 129(2):262e–274e. doi:10.1097/PRS.0b013e31823aeb3b
Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, Rumbaugh KP (2011) An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 6(11):e27317. doi:10.1371/journal.pone.0027317
Gurjala AN, Geringer MR, Seth AK, Hong SJ, Smeltzer MS, Galiano RD, Leung KP, Mustoe TA (2011) Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen 19(3):400–410. doi:10.1111/j.1524-475X.2011.00690.x
Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, Fleckman P, Olerud JE (2010) Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen 18(5):467–477
Wolcott RD, Rhoads DD (2008) A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care 17(4):145–148, 150–142, 154–145
Wu K, Kah, Y. (2008) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol UNIT 5.47 doi:10.1002/0471141755.ph0547s40
Lanng S, Thorsteinsson B, Nerup J, Koch C (1994) Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatr 83(8):849–853
Gauglitz GG, Toliver-Kinsky TE, Williams FN, Song J, Cui W, Herndon DN, Jeschke MG (2010) Insulin increases resistance to burn wound infection-associated sepsis. Crit Care Med 38(1):202–208. doi:10.1097/CCM.0b013e3181b43236
Jeschke MG, Kulp GA, Kraft R, Finnerty CC, Mlcak R, Lee JO, Herndon DN (2010) Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med 182(3):351–359. doi:10.1164/rccm.201002-0190OC
Chai Y, Kolter R, Losick R (2009) A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and its involvement in biofilm formation. J Bacteriol 191(8):2423–2430. doi:10.1128/JB.01464-08
Pelegrinelli FF, Thirone AC, Gasparetti AL, Araujo EP, Velloso LA, Saad MJ (2001) Early steps of insulin action in the skin of intact rats. J Invest Dermatol 117(4):971–976. doi:10.1046/j.0022-202x.2001.01473.x
Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S (2001) Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86(7):3257–3265
Dandona P, Chaudhuri A, Mohanty P, Ghanim H (2007) Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care 10(4):511–517. doi:10.1097/MCO.0b013e3281e38774
Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P (2008) Acute modulation of toll-like receptors by insulin. Diabetes Care 31(9):1827–1831. doi:10.2337/dc08-0561
Kidd LB, Schabbauer GA, Luyendyk JP, Holscher TD, Tilley RE, Tencati M, Mackman N (2008) Insulin activation of the phosphatidylinositol 3-kinase/protein kinase B (Akt) pathway reduces lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther 326(1):348–353. doi:10.1124/jpet.108.138891
Acknowledgments
This study was supported by American Diabetes Association research grant #1-08-RA-165 to KPR. KD and UT were supported by a Howard Hughes Medical Institute grant through the Undergraduate Science Education Program to Texas Tech University. MJW was supported by a grant to the Vermont Center for Immunology and Infectious Disease from the National Center for Research Resources (5P20RR021905-07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watters, C., DeLeon, K., Trivedi, U. et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol 202, 131–141 (2013). https://doi.org/10.1007/s00430-012-0277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-012-0277-7