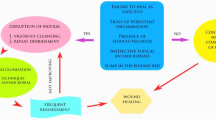
Abstract
Purpose of Review
To provide an up-to-date overview of recent developments in diagnostic methods and therapeutic approaches for chronic wound biofilms and pathogenic microbiota.
Recent Findings
Biofilm infections are one of the major contributors to impaired wound healing in chronic wounds, including diabetic foot ulcers, venous leg ulcers, pressure ulcers, and nonhealing surgical wounds. As an organized microenvironment commonly including multiple microbial species, biofilms develop and persist through methods that allow evasion from host immune response and antimicrobial treatments. Suppression and reduction of biofilm infection have been demonstrated to improve wound healing outcomes. However, chronic wound biofilms are a challenge to treat due to limited methods for accurate, accessible clinical identification and the biofilm’s protective properties against therapeutic agents. Here we review recent approaches towards visual markers for less invasive, enhanced biofilm detection in the clinical setting. We outline progress in wound care treatments including investigation of their antibiofilm effects, such as with hydrosurgical and ultrasound debridement, negative pressure wound therapy with instillation, antimicrobial peptides, nanoparticles and nanocarriers, electroceutical dressings, and phage therapy.
Summary
Current evidence for biofilm-targeted treatments has been primarily conducted in preclinical studies, with limited clinical investigation for many therapies. Improved identification, monitoring, and treatment of biofilms require expansion of point-of-care visualization methods and increased evaluation of antibiofilm therapies in robust clinical trials.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Versey Z, da Cruz Nizer WS, Russell E, Zigic S, DeZeeuw KG, Marek JE, et al. Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol. 2021;12: 648554. https://doi.org/10.3389/fimmu.2021.648554.
Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, et al. Global Wound Biofilm Expert P. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25(5):744–57. https://doi.org/10.1111/wrr.12590.
Kim JH, Yang B, Tedesco A, Lebig EGD, Ruegger PM, Xu K, et al. High levels of oxidative stress and skin microbiome are critical for initiation and development of chronic wounds in diabetic mice. Sci Rep. 2019;9(1):19318. https://doi.org/10.1038/s41598-019-55644-3.
Tipton CD, Wolcott RD, Sanford NE, Miller C, Pathak G, Silzer TK, et al. Patient genetics is linked to chronic wound microbiome composition and healing. PLoS Pathog. 2020;16(6): e1008511. https://doi.org/10.1371/journal.ppat.1008511.
Di Domenico EG, Cavallo I, Bordignon V, Prignano G, Sperduti I, Gurtner A, et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of atopic dermatitis. Sci Rep. 2018;8(1):9573. https://doi.org/10.1038/s41598-018-27421-1.
Platsidaki E, Dessinioti C. Recent advances in understanding propionibacterium acnes ( cutibacterium acnes) in acne. F1000Res. 2018;7. https://doi.org/10.12688/f1000research.15659.1
Ring HC, Bay L, Nilsson M, Kallenbach K, Miller IM, Saunte DM, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176(4):993–1000. https://doi.org/10.1111/bjd.15007.
Tomic-Canic M, Burgess JL, O’Neill KE, Strbo N, Pastar I. Skin microbiota and its interplay with wound healing. Am J Clin Dermatol. 2020;21(Suppl 1):36–43. https://doi.org/10.1007/s40257-020-00536-w.
Grogan MD, Bartow-McKenney C, Flowers L, Knight SAB, Uberoi A, Grice EA. Research techniques made simple: profiling the skin microbiota. J Invest Dermatol. 2019;139(4):747–52 e1. https://doi.org/10.1016/j.jid.2019.01.024.
Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62(3):923–30. https://doi.org/10.2337/db12-0771.
Kalan LR, Meisel JS, Loesche MA, Horwinski J, Soaita I, Chen X, et al. Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe. 2019;25(5):641–55 e5. https://doi.org/10.1016/j.chom.2019.03.006.
Verbanic S, Shen Y, Lee J, Deacon JM, Chen IA. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes. 2020;6(1):21. https://doi.org/10.1038/s41522-020-0130-5.
•• Loesche M, Gardner SE, Kalan L, Horwinski J, Zheng Q, Hodkinson BP, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237–44. https://doi.org/10.1016/j.jid.2016.08.009. The first study linking microbiota composition to healing outcomes in patients with chronic wounds.
Dunyach-Remy C, Salipante F, Lavigne JP, Brunaud M, Demattei C, Yahiaoui-Martinez A, et al. Pressure ulcers microbiota dynamics and wound evolution. Sci Rep. 2021;11(1):18506. https://doi.org/10.1038/s41598-021-98073-x.
Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, et al. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio. 2016;7(5). https://doi.org/10.1128/mBio.01058-16.
•• Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol. 2011;11:143. https://doi.org/10.1186/1471-2180-11-143. Biofilm cultures of Staphylococcus aureus induced a distinct inflammatory response in human keratinocytes that may contribute to chronicity of non healing wounds.
Jeffery Marano R, Jane Wallace H, Wijeratne D, William Fear M, San Wong H, O’Handley R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci Rep. 2015;5:13296. https://doi.org/10.1038/srep13296.
Pestrak MJ, Chaney SB, Eggleston HC, Dellos-Nolan S, Dixit S, Mathew-Steiner SS, et al. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog. 2018;14(2): e1006842. https://doi.org/10.1371/journal.ppat.1006842.
Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, et al. Deregulated immune cell recruitment orchestrated by foxm1 impairs human diabetic wound healing. Nat Commun. 2020;11(1):4678. https://doi.org/10.1038/s41467-020-18276-0.
Roy S, Santra S, Das A, Dixith S, Sinha M, Ghatak S, et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann Surg. 2020;271(6):1174–85. https://doi.org/10.1097/SLA.0000000000003053.
Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18S-28S. https://doi.org/10.1097/PRS.0000000000002682.
Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol. 2014;233(4):331–43. https://doi.org/10.1002/path.4360.
Pastar I, Marjanovic J, Stone RC, Chen V, Burgess JL, Mervis JS, et al. Epigenetic regulation of cellular functions in wound healing. Exp Dermatol. 2021. https://doi.org/10.1111/exd.14325.
Strbo N, Pastar I, Romero L, Chen V, Vujanac M, Sawaya AP, et al. Single cell analyses reveal specific distribution of anti-bacterial molecule perforin-2 in human skin and its modulation by wounding and Staphylococcus aureus infection. Exp Dermatol. 2019;28(3):225–32. https://doi.org/10.1111/exd.13870.
Pastar I, Sawaya AP, Marjanovic J, Burgess JL, Strbo N, Rivas KE, et al. Intracellular Staphylococcus aureus triggers pyroptosis and contributes to inhibition of healing due to perforin-2 suppression. J Clin Invest. 2021. https://doi.org/10.1172/JCI133727.
Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26(1):20–5. https://doi.org/10.12968/jowc.2017.26.1.20.
Johani K, Malone M, Jensen S, Gosbell I, Dickson H, Hu H, et al. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int Wound J. 2017;14(6):1160–9. https://doi.org/10.1111/iwj.12777.
Oates A, Bowling FL, Boulton AJ, Bowler PG, Metcalf DG, McBain AJ. The visualization of biofilms in chronic diabetic foot wounds using routine diagnostic microscopy methods. J Diabetes Res. 2014;2014: 153586. https://doi.org/10.1155/2014/153586.
Gwynne L, Williams GT, Yan KC, Patenall BL, Gardiner JE, He XP, et al. Tcf-alp: a fluorescent probe for the selective detection of staphylococcus bacteria and application in “smart” wound dressings. Biomater Sci. 2021;9(12):4433–9. https://doi.org/10.1039/d0bm01918f.
Wu YF, Lee TY, Liao WT, Chuan HH, Cheng NC, Cheng CM. Rapid detection of biofilm with modified alcian blue staining: in-vitro protocol improvement and validation with clinical cases. Wound Repair Regen. 2020;28(6):834–43. https://doi.org/10.1111/wrr.12845.
Lopez AJ, Jones LM, Reynolds L, Diaz RC, George IK, Little W, et al. Detection of bacterial fluorescence from in vivo wound biofilms using a point-of-care fluorescence imaging device. Int Wound J. 2021;18(5):626–38. https://doi.org/10.1111/iwj.13564.
Raizman R, Little W, Smith AC. Rapid diagnosis of pseudomonas aeruginosa in wounds with point-of-care fluorescence imaing. Diagnostics (Basel). 2021;11(2). https://doi.org/10.3390/diagnostics11020280.
•• Le L, Baer M, Briggs P, Bullock N, Cole W, DiMarco D, et al. Diagnostic accuracy of point-of-care fluorescence imaging for the detection of bacterial burden in wounds: results from the 350-patient fluorescence imaging assessment and guidance trial. Adv Wound Care (New Rochelle). 2021;10(3):123–36. https://doi.org/10.1089/wound.2020.1272. A recent prospective, multi-center controlled study of 350 patients with chronic wounds revealed traditional clinical assessment failed to predict bacterial loads in 85% of infected wounds, while fluorescence imaging significantly increased bacterial detection and influenced patient care across all wound types.
Farhan N, Jeffery S. Utility of moleculight i: X for managing bacterial burden in pediatric burns. J Burn Care Res. 2020;41(2):328–38. https://doi.org/10.1093/jbcr/irz167.
Pastar I, Cao T, Sawaya A, Liang L, Glinos G, Drakulich S, et al. Preclinical models for wound-healing studies. In: Marques A, Reis R, Pirraco R, Cerqueira M, editors. Skin tissue models: Elsevier, Inc; 2017. pp. 223–51.
Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17(3):354–9. https://doi.org/10.1111/j.1524-475X.2009.00489.x.
Redman WK, Welch GS, Rumbaugh KP. Assessing biofilm dispersal in murine wounds. J Vis Exp. 2021(174). https://doi.org/10.3791/62136.
Huang J, Fan Q, Guo M, Wu M, Wu S, Shen S, et al. Octenidine dihydrochloride treatment of a meticillin-resistant Staphylococcus aureus biofilm-infected mouse wound. J Wound Care. 2021;30(2):106–14. https://doi.org/10.12968/jowc.2021.30.2.106.
Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res. 2014;2014: 562625. https://doi.org/10.1155/2014/562625.
Chien S. Ischemic rabbit ear model created by minimally invasive surgery. Wound Repair Regen. 2007;15(6):928–35. https://doi.org/10.1111/j.1524-475X.2007.00285.x.
Seth AK, Zhong A, Nguyen KT, Hong SJ, Leung KP, Galiano RD, et al. Impact of a novel, antimicrobial dressing on in vivo, pseudomonas aeruginosa wound biofilm: quantitative comparative analysis using a rabbit ear model. Wound Repair Regen. 2014;22(6):712–9. https://doi.org/10.1111/wrr.12232.
Park E, Long SA, Seth AK, Geringer M, Xu W, Chavez-Munoz C, et al. The use of desiccation to treat staphylococcus aureus biofilm-infected wounds. Wound Repair Regen. 2016;24(2):394–401. https://doi.org/10.1111/wrr.12379.
Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9(2):66–76. https://doi.org/10.1046/j.1524-475x.2001.00066.x.
Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56(1):127–38. https://doi.org/10.1093/ilar/ilv016.
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445–64. https://doi.org/10.1089/wound.2013.0473.
Nusbaum AG, Gil J, Rippy MK, Warne B, Valdes J, Claro A, et al. Effective method to remove wound bacteria: comparison of various debridement modalities in an in vivo porcine model. J Surg Res. 2012;176(2):701–7. https://doi.org/10.1016/j.jss.2011.11.1040.
Schwartz JA, Goss SG, Facchin F, Avdagic E, Lantis JC. Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care. 2014;23(9):S4, S6, S8 passim. https://doi.org/10.12968/jowc.2014.23.Sup9.S4.
Kim PJ, Attinger CE, Bigham T, Hagerty R, Platt S, Anghel E, et al. Clinic-based debridement of chronic ulcers has minimal impact on bacteria. Wounds. 2018;30(5):114–9.
Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care. 2010;19(8):320–8. https://doi.org/10.12968/jowc.2010.19.8.77709.
Bowling FL, Stickings DS, Edwards-Jones V, Armstrong DG, Boulton AJ. Hydrodebridement of wounds: effectiveness in reducing wound bacterial contamination and potential for air bacterial contamination. J Foot Ankle Res. 2009;2:13. https://doi.org/10.1186/1757-1146-2-13.
Liu J, Ko JH, Secretov E, Huang E, Chukwu C, West J, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12(4):456–61. https://doi.org/10.1111/iwj.12137.
Sonnergren HH, Strombeck L, Aldenborg F, Faergemann J. Aerosolized spread of bacteria and reduction of bacterial wound contamination with three different methods of surgical wound debridement: A pilot study. J Hosp Infect. 2013;85(2):112–7. https://doi.org/10.1016/j.jhin.2013.05.011.
Voigt J, Wendelken M, Driver V, Alvarez OM. Low-frequency ultrasound (20–40 khz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta-analysis of eight randomized controlled trials. Int J Low Extrem Wounds. 2011;10(4):190–9. https://doi.org/10.1177/1534734611424648.
Kataoka Y, Kunimitsu M, Nakagami G, Koudounas S, Weller CD, Sanada H. Effectiveness of ultrasonic debridement on reduction of bacteria and biofilm in patients with chronic wounds: a scoping review. Int Wound J. 2021;18(2):176–86. https://doi.org/10.1111/iwj.13509.
Chang YR, Perry J, Cross K. Low-frequency ultrasound debridement in chronic wound healing: a systematic review of current evidence. Plast Surg (Oakv). 2017;25(1):21–6. https://doi.org/10.1177/2292550317693813.
Vig S, Dowsett C, Berg L, Caravaggi C, Rome P, Birke-Sorensen H, et al. Evidence-based recommendations for the use of negative pressure wound therapy in chronic wounds: steps towards an international consensus. J Tissue Viability. 2011;20 Suppl 1:S1–18. https://doi.org/10.1016/j.jtv.2011.07.002.
Li T, Wang G, Yin P, Li Z, Zhang L, Tang P. Adaptive expression of biofilm regulators and adhesion factors of Staphylococcus aureus during acute wound infection under the treatment of negative pressure wound therapy in vivo. Exp Ther Med. 2020;20(1):512–20. https://doi.org/10.3892/etm.2020.8679.
Kim PJ, Attinger CE, Crist BD, Gabriel A, Galiano RD, Gupta S, et al. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds. 2015;27(12):S2–19.
Patmo AS, Krijnen P, Tuinebreijer WE, Breederveld RS. The effect of vacuum-assisted closure on the bacterial load and type of bacteria: a systematic review. Adv Wound Care (New Rochelle). 2014;3(5):383–9. https://doi.org/10.1089/wound.2013.0510.
•• Goss SG, Schwartz JA, Facchin F, Avdagic E, Gendics C, Lantis JC, 2nd. Negative pressure wound therapy with instillation (npwti) better reduces post-debridement bioburden in chronically infected lower extremity wounds than npwt alone. J Am Coll Clin Wound Spec. 2012;4(4):74–80. https://doi.org/10.1016/j.jccw.2014.02.001. Prospective pilot clinical study showing NPWT with instillation decreased bacterial loads at a clinically significant level, while NPWT did not.
Yang C, Goss SG, Alcantara S, Schultz G, Lantis Ii JC. Effect of negative pressure wound therapy with instillation on bioburden in chronically infected wounds. Wounds. 2017;29(8):240–6.
Jeong HS, Lee BH, Lee HK, Kim HS, Moon MS, Suh IS. Negative pressure wound therapy of chronically infected wounds using 1% acetic acid irrigation. Arch Plast Surg. 2015;42(1):59–67. https://doi.org/10.5999/aps.2015.42.1.59.
Kim PJ, Lavery LA, Galiano RD, Salgado CJ, Orgill DP, Kovach SJ, et al. The impact of negative-pressure wound therapy with instillation on wounds requiring operative debridement: pilot randomised, controlled trial. Int Wound J. 2020;17(5):1194–208. https://doi.org/10.1111/iwj.13424.
Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10(Suppl 1):56–60. https://doi.org/10.1111/iwj.12176.
Serena TE, Jalodi O, Serena L, Patel K, Mynti M. Evaluation of the combination of a biofilm-disrupting agent and negative pressure wound therapy: a case series. J Wound Care. 2021;30(1):9–14. https://doi.org/10.12968/jowc.2021.30.1.9.
Hahn HM, Lee IJ, Woo KJ, Park BY. Silver-impregnated negative-pressure wound therapy for the treatment of lower-extremity open wounds: a prospective randomized clinical study. Adv Skin Wound Care. 2019;32(8):370–7. https://doi.org/10.1097/01.ASW.0000569116.59534.a6.
Chung PY, Khanum R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J Microbiol Immunol Infect. 2017;50(4):405–10. https://doi.org/10.1016/j.jmii.2016.12.005.
Spohn R, Daruka L, Lazar V, Martins A, Vidovics F, Grezal G, et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat Commun. 2019;10(1):4538. https://doi.org/10.1038/s41467-019-12364-6.
Kubicek-Sutherland JZ, Lofton H, Vestergaard M, Hjort K, Ingmer H, Andersson DI. Antimicrobial peptide exposure selects for staphylococcus aureus resistance to human defence peptides. J Antimicrob Chemother. 2017;72(1):115–27. https://doi.org/10.1093/jac/dkw381.
Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A. 2015;112(24):7569–74. https://doi.org/10.1073/pnas.1506207112.
Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. Human host defense peptide ll-37 prevents bacterial biofilm formation. Infect Immun. 2008;76(9):4176–82. https://doi.org/10.1128/IAI.00318-08.
Haisma EM, Goblyos A, Ravensbergen B, Adriaans AE, Cordfunke RA, Schrumpf J, et al. Antimicrobial peptide p60.4ac-containing creams and gel for eradication of methicillin-resistant Staphylococcus aureus from cultured skin and airway epithelial surfaces. Antimicrob Agents Chemother. 2016;60(7):4063–72. https://doi.org/10.1128/AAC.03001-15.
Lora-Tamayo J, Murillo O, Ariza J. Clinical use of colistin in biofilm-associated infections. Adv Exp Med Biol. 2019;1145:181–95. https://doi.org/10.1007/978-3-030-16373-0_13.
Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis. 2008;47(12):1537–45. https://doi.org/10.1086/593185.
Starr CG, He J, Wimley WC. Host cell interactions are a significant barrier to the clinical utility of peptide antibiotics. ACS Chem Biol. 2016;11(12):3391–9. https://doi.org/10.1021/acschembio.6b00843.
Sonesson A, Przybyszewska K, Eriksson S, Morgelin M, Kjellstrom S, Davies J, et al. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci Rep. 2017;7(1):8689. https://doi.org/10.1038/s41598-017-08046-2.
Golla RM, Mishra B, Dang X, Lakshmaiah Narayana J, Li A, Xu L, et al. Resistome of Staphylococcus aureus in response to human cathelicidin ll-37 and its engineered antimicrobial peptides. ACS Infect Dis. 2020;6(7):1866–81. https://doi.org/10.1021/acsinfecdis.0c00112.
Lam SJ, O’Brien-Simpson NM, Pantarat N, Sulistio A, Wong EH, Chen YY, et al. Combating multidrug-resistant gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol. 2016;1(11):16162. https://doi.org/10.1038/nmicrobiol.2016.162.
Peulen TO, Wilkinson KJ. Diffusion of nanoparticles in a biofilm. Environ Sci Technol. 2011;45(8):3367–73. https://doi.org/10.1021/es103450g.
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–84. https://doi.org/10.1016/j.msec.2014.08.031.
Mekkawy AI, El-Mokhtar MA, Nafady NA, Yousef N, Hamad MA, El-Shanawany SM, et al. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: effect of surface coating and loading into hydrogels. Int J Nanomedicine. 2017;12:759–77. https://doi.org/10.2147/IJN.S124294.
Finley PJ, Peterson A, Huckfeldt RE. The prevalence of phenotypic silver resistance in clinical isolates. Wounds. 2013;25(4):84–8.
Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129(10):2463–9. https://doi.org/10.1038/jid.2009.95.
Wang M, Lai X, Shao L, Li L. Evaluation of immunoresponses and cytotoxicity from skin exposure to metallic nanoparticles. Int J Nanomedicine. 2018;13:4445–59. https://doi.org/10.2147/IJN.S170745.
Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23. https://doi.org/10.1016/j.jconrel.2014.03.055.
Teirlinck E, Xiong R, Brans T, Forier K, Fraire J, Van Acker H, et al. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat Commun. 2018;9(1):4518. https://doi.org/10.1038/s41467-018-06884-w.
Rout B, Liu CH, Wu WC. Photosensitizer in lipid nanoparticle: a nano-scaled approach to antibacterial function. Sci Rep. 2017;7(1):7892. https://doi.org/10.1038/s41598-017-07444-w.
Li X, Liu Z, Liu H, Chen X, Liu Y, Tan H. Photodynamic inactivation of fibroblasts and inhibition of staphylococcus epidermidis adhesion and biofilm formation by toluidine blue o. Mol Med Rep. 2017;15(4):1816–22. https://doi.org/10.3892/mmr.2017.6184.
•• Morley S, Griffiths J, Philips G, Moseley H, O'Grady C, Mellish K, et al. Phase iia randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol. 2013;168(3):617–24. https://doi.org/10.1111/bjd.12098. The first controlled study of photodynamic therapy (PDT) in chronic wounds. This blinded, randomized placebo-controlled phase IIa trial confirmed PDT reduced bacterial load immediately post-treatment, with acceptable safety profile and improved healing outcomes.
Hu D, Li H, Wang B, Ye Z, Lei W, Jia F, et al. Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant staphylococcus aureus biofilm. ACS Nano. 2017;11(9):9330–9. https://doi.org/10.1021/acsnano.7b04731.
Nguyen TK, Duong HT, Selvanayagam R, Boyer C, Barraud N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci Rep. 2015;5:18385. https://doi.org/10.1038/srep18385.
Devlin H, Fulaz S, Hiebner DW, O’Gara JP, Casey E. Enzyme-functionalized mesoporous silica nanoparticles to target Staphylococcus aureus and disperse biofilms. Int J Nanomedicine. 2021;16:1929–42. https://doi.org/10.2147/IJN.S293190.
Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14(6):449–59. https://doi.org/10.1007/s40257-013-0046-4.
Administration USFaD. 510(k) Summary for derma sciences medihoney dressings with active manuka honey. 2008.
Lu J, Turnbull L, Burke CM, Liu M, Carter DA, Schlothauer RC, et al. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ. 2014;2: e326. https://doi.org/10.7717/peerj.326.
Kot B, Sytykiewicz H, Sprawka I, Witeska M. Effect of manuka honey on biofilm-associated genes expression during methicillin-resistant staphylococcus aureus biofilm formation. Sci Rep. 2020;10(1):13552. https://doi.org/10.1038/s41598-020-70666-y.
Liu MY, Cokcetin NN, Lu J, Turnbull L, Carter DA, Whitchurch CB, et al. Rifampicin-manuka honey combinations are superior to other antibiotic-manuka honey combinations in eradicating staphylococcus aureus biofilms. Front Microbiol. 2017;8:2653. https://doi.org/10.3389/fmicb.2017.02653.
Abd El-Malek FF, Yousef AS, El-Assar SA. Hydrogel film loaded with new formula from manuka honey for treatment of chronic wound infections. J Glob Antimicrob Resist. 2017;11:171–6. https://doi.org/10.1016/j.jgar.2017.08.007.
Frydman GH, Olaleye D, Annamalai D, Layne K, Yang I, et al. Manuka honey microneedles for enhanced wound healing and the prevention and/or treatment of methicillin-resistant staphylococcus aureus (mrsa) surgical site infection. Sci Rep. 2020;10(1):13229. https://doi.org/10.1038/s41598-020-70186-9.
Wang C, Guo M, Zhang N, Wang G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: a systematic review and meta-analysis. Complement Ther Clin Pract. 2019;34:123–31. https://doi.org/10.1016/j.ctcp.2018.09.004.
Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-oh kinase-gamma and pten. Nature. 2006;442(7101):457–60. https://doi.org/10.1038/nature04925.
Banerjee J, Das Ghatak P, Roy S, Khanna S, Sequin EK, Bellman K, et al. Improvement of human keratinocyte migration by a redox active bioelectric dressing. PLoS ONE. 2014;9(3): e89239. https://doi.org/10.1371/journal.pone.0089239.
Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527(7576):59–63. https://doi.org/10.1038/nature15709.
Humphries J, Xiong L, Liu J, Prindle A, Yuan F, Arjes HA, et al. Species-independent attraction to biofilms through electrical signaling. Cell. 2017;168(1–2):200–9 e12. https://doi.org/10.1016/j.cell.2016.12.014.
Administration USFaD. Procellera (for professional use) 510(k) summary of safety and effectiveness. 2008.
Roy S, Prakash S, Mathew-Steiner SS, Das Ghatak P, Lochab V, Jones TH, et al. Disposable patterned electroceutical dressing (ped-10) is safe for treatment of open clinical chronic wounds. Adv Wound Care (New Rochelle). 2019;8(4):149–59. https://doi.org/10.1089/wound.2018.0915.
Blount AL, Foster S, Rapp DA, Wilcox R. The use of bioelectric dressings in skin graft harvest sites: a prospective case series. J Burn Care Res. 2012;33(3):354–7. https://doi.org/10.1097/BCR.0b013e31823356e4.
Atkin L, Bucko Z, Conde Montero E, Cutting K, Moffatt C, Probst A, et al. Implementing timers: the race against hard-to-heal wounds. J Wound Care. 2019;23(Sup3a):S1-S50. https://doi.org/10.12968/jowc.2019.28.Sup3a.S1.
Malone M, Radzieta M, Schwarzer S, Jensen SO, Lavery LA. Efficacy of a topical concentrated surfactant gel on microbial communities in non-healing diabetic foot ulcers with chronic biofilm infections: a proof-of-concept study. Int Wound J. 2021;18(4):457–66. https://doi.org/10.1111/iwj.13546.
Kim D, Namen Ii W, Moore J, Buchanan M, Hayes V, Myntti MF, et al. Clinical assessment of a biofilm-disrupting agent for the management of chronic wounds compared with standard of care: A therapeutic approach. Wounds. 2018;30(5):120–30.
Wolcott R. Disrupting the biofilm matrix improves wound healing outcomes. J Wound Care. 2015;24(8):366–71. https://doi.org/10.12968/jowc.2015.24.8.366.
Seth AK, Geringer MR, Nguyen KT, Agnew SP, Dumanian Z, Galiano RD, et al. Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: A new approach to chronic wound care. Plast Reconstr Surg. 2013;131(2):225–34. https://doi.org/10.1097/PRS.0b013e31827e47cd.
Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, et al. Design of a broad-range bacteriophage cocktail that reduces pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother. 2018;62(6). https://doi.org/10.1128/AAC.02573-17.
Holguin AV, Rangel G, Clavijo V, Prada C, Mantilla M, Gomez MC, et al. Phage phipan70, a putative temperate phage, controls pseudomonas aeruginosa in planktonic, biofilm and burn mouse model assays. Viruses. 2015;7(8):4602–23. https://doi.org/10.3390/v7082835.
Oliveira A, Sousa JC, Silva AC, Melo LDR, Sillankorva S. Chestnut honey and bacteriophage application to control pseudomonas aeruginosa and escherichia coli biofilms: evaluation in an ex vivo wound model. Front Microbiol. 2018;9:1725. https://doi.org/10.3389/fmicb.2018.01725.
•• Jault P, Leclerc T, Jennes S, Pirnay JP, Que YA, Resch G, et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by pseudomonas aeruginosa (phagoburn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis. 2019;19(1):35–45. https://doi.org/10.1016/S1473-3099(18)30482-1. A randomized clinical trial for bacteriophage therapy in infected wounds demonstrating clinical potential and challenges of bacteriophage methods in reducing wound bacterial load.
Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. Methods Mol Biol. 2018;1693:159–70. https://doi.org/10.1007/978-1-4939-7395-8_14.
Patel DR, Bhartiya SK, Kumar R, Shukla VK, Nath G. Use of customized bacteriophages in the treatment of chronic nonhealing wounds: a prospective study. Int J Low Extrem Wounds. 2021;20(1):37–46. https://doi.org/10.1177/1534734619881076.
Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 2019;363(6434). https://doi.org/10.1126/science.aat9691.
Davis SC, Pastar I. Reply to “questioning the use of an acute porcine wound model to assess anti-biofilm activity of dressings.” Wound Repair Regen. 2020;28(3):429–30. https://doi.org/10.1111/wrr.12795.
Nakagami G, Schultz G, Kitamura A, Minematsu T, Akamata K, Suga H, et al. Rapid detection of biofilm by wound blotting following sharp debridement of chronic pressure ulcers predicts wound healing: a preliminary study. Int Wound J. 2020;17(1):191–6. https://doi.org/10.1111/iwj.13256.
Rennie MY, Lindvere-Teene L, Tapang K, Linden R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: a clinical study. J Wound Care. 2017;26(8):452–60. https://doi.org/10.12968/jowc.2017.26.8.452
Acknowledgements
We are thankful to all current and past lab members for continuous inspiration and support. Please note that due to space limitations some relevant work from the field could not be cited. The figure in this manuscript was created using biorender.com. This work is in part supported by R01NR015649, U01DK119085, U24DK122927, and R01AR073614 (MTC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
IP research is in part supported by Next Science. MTC research is in part supported by Organogenis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Wound Care
Rights and permissions
About this article
Cite this article
Chen, V., Burgess, J.L., Verpile, R. et al. Novel Diagnostic Technologies and Therapeutic Approaches Targeting Chronic Wound Biofilms and Microbiota. Curr Derm Rep 11, 60–72 (2022). https://doi.org/10.1007/s13671-022-00354-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-022-00354-9