Abstract
Consumer wearables and health monitors, internet-based health and cognitive assessments, and at-home biosample (e.g., saliva and capillary blood) collection kits are increasingly used by public health researchers for large population-based studies without requiring intensive in-person visits. Alongside reduced participant time burden, remote and virtual data collection allows the participation of individuals who live long distances from hospital or university research centers, or who lack access to transportation. Unfortunately, studies that include magnetic resonance neuroimaging are challenging to perform remotely given the infrastructure requirements of MRI scanners, and, as a result, they often omit socially, economically, and educationally disadvantaged individuals. Lower field strength systems (< 100 mT) offer the potential to perform neuroimaging at a participant’s home, enabling more accessible and equitable research. Here we report the first use of a low-field MRI “scan van” with an online assessment of paired-associate learning (PAL) to examine associations between brain morphometry and verbal memory performance. In a sample of 67 individuals, 18–93 years of age, imaged at or near their home, we show expected white and gray matter volume trends with age and find significant (p < 0.05 FWE) associations between PAL performance and hippocampus, amygdala, caudate, and thalamic volumes. High-quality data were acquired in 93% of individuals, and at-home scanning was preferred by all individuals with prior MRI at a hospital or research setting. Results demonstrate the feasibility of remote neuroimaging and cognitive data collection, with important implications for engaging traditionally under-represented communities in neuroimaging research.
Similar content being viewed by others
Introduction
Neuroimaging has provided salient information on the changing brain tissue macro and micro-structure, cortical and sub-cortical morphology and morphometry, and functional connectivity across the lifespan. Studies of volumetric change describe a non-linear pattern characterized by rapid growth of the brain’s white and gray matter throughout infancy and childhood, peaking in the second to fourth decades of life, followed by a slow but progressive decline throughout adulthood (Westlye et al. 2010; Taki et al. 2011; Terribilli et al. 2011; Goodro et al. 2012; Long et al. 2012; Ziegler et al. 2012; Narvacan et al. 2017; Bethlehem et al. 2022). Tissue-wise and regional differences exist, with cortical gray matter reaching its maximal value during adolescence while global white matter volume is maximal between 30 and 40 years of age (Taki et al. 2011; Ziegler et al. 2012; Bethlehem et al. 2022). Subcortical structures, similarly, follow differential growth trajectories, with peak values occurring throughout the second decade of life (Long et al. 2012; Narvacan et al. 2017). Overall, patterns are generally preserved between males and females (Bethlehem et al. 2022; Lenroot et al. 2007), though absolute volume is, on average, greater in males in large part due to their larger physical body and head size.
Patterns of brain change across the lifespan have further been associated with emerging and receding cognitive skills and abilities. Volume reductions in memory-related regions (e.g., hippocampus) have been associated with age-related memory changes among otherwise healthy older adults, as well as in mild cognitive impairment and Alzheimer’s disease (Fox et al. 1996; Shi et al. 2009; Aljondi et al. 2019; Armstrong et al. 2020). More generally and beyond memory function, global and regional brain volumes have been associated with differential executive functioning skills (Aljondi et al. 2019; Elderkin-Thompson et al. 2008; Elderkin-Thompson et al. 2009), processing speed (Anstey et al. 2007; Walhovd and Fjell 2007), and general intelligence (Fling et al. 2011; Cox et al. 2019).
Despite the utility of neuroimaging to the study of healthy aging and neurodegenerative disorders, MRI studies are expensive and often limited to specialized university imaging centers or larger research hospitals. As consequence, they typically have relatively small sample study sizes (n < 30) and rely on populations of convenience, i.e., geographically proximal participants who are able to travel independently or have nearby family members or other support. These factors can bias the study population toward particular sociodemographic phenotypes (e.g., higher socioeconomic and/or educational backgrounds, individuals living independently with lower disease burden, etc.) that may affect the generalizability of findings and conclusions. Large-scale neuroimaging initiatives such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (Weiner et al. 2013), the Human and Lifespan Connectome Projects (Bookheimer et al. 2019; Elam et al. 2021), and the UK Biobank (Alfaro-Almagro et al. 2018) aim to provide study sizes large enough to avoid potential biases (or enable direct modeling of them). These large studies, however, are financially expensive and logistically complex, and still require participating individuals to travel to centralized imaging and research centers.
Over the past 5–10 years, internet and tablet-based tools have made scalable and remote cognitive assessments feasible (Hooyman et al. 2021; Thompson et al. 2022; Tsiakiri 2022). This trend toward remote assessment has been further accelerated by the COVID-19 pandemic, which forced many research and healthcare centers to seek reliable and reproducible online alternatives to traditional in-person visits and assessments (Thompson et al. 2022; Geddes et al. 2020; Hill et al. 2021). These tools offer the potential to reach beyond the traditional study populations and include participants from a wide range of geographic, demographic, and socioeconomic backgrounds.
MindCrowd (Talboom et al. 2019, 2021; Huentelman et al. 2020; Rodrigo et al. 2021) is one such accessible and easy-to-use web-based platform for cognitive and demographic assessment, designed specifically to overcome challenges with small sample-size studies and to increase inclusive and diverse participation. Participants over 18 years of age can anonymously provide general background personal information (e.g., age, biological sex, education attainment), as well as more specific and granular sociodemographic and health history data (e.g., race, ethnicity, number of daily prescription medications, and first-degree family history of dementia). Participants can optionally provide identifiable name and residential address information, and indicate a preference or willingness to provide biosample collections and participate in ancillary studies. Cognitive assessment on MindCrowd consists of simple visual reaction time (svRT) and paired-associates learning (PAL) tasks–quick and sensitive tests of processing speed and associative episodic memory function, respectively. Past work has shown these cognitive functions are often affected in the earliest stages of cognitive impairment and Alzheimer’s Disease (AD) (Phillips et al. 2013; Andriuta et al. 2019; Baker et al. 2019), and also reflect the general decline in cognitive performance in healthy aging (Lowndes et al. 2008; Jackson et al. 2012). The ability to capture data from hundreds of thousands of participants at a relatively low cost and without intensive in-person visits has allowed the team behind MindCrowd to investigate the impact of diverse family, medical history, and genetic factors on cognitive performance across the adult lifespan (Talboom, et al. 2019, 2021), and to identify potential cases of previously undiagnosed cases of cognitive impairment and dementia (Rodrigo et al. 2021).
While reliable remote and internet-based cognitive assessments are becoming increasingly common, remote collection of MRI data has been impracticable due to the size and weight of common 1.5 and 3 Tesla (T) systems, as well as their electrical requirements, and helium and maintenance needs. Though semi-trailer 18-wheeler mounted 1.5 T systems are broadly available throughout North America, Europe, and Asia, they share the size, weight, and electrical needs of their sited brethren, and are designed for institutional use as adjuncts to static systems installed at hospitals or clinics, or as semi-permeant solutions for smaller institutions. These systems require specially installed concrete parking pads and high voltage electrical supplies and are not designed for use at a participant’s home. However, advancements in MRI systems that operate at lower magnetic field strength (i.e., less than 100mT) with permanent or resistive magnet arrays present an alternative to conventional systems with the possibility of enabling “residential MRI”—truly remote neuroimaging performed at a participant’s home, assisted living facility, or other convenient and nearby location (e.g., library, shopping center, etc.) (Deoni et al. 2022a). While lower field systems are not replacements for higher field strength scanners, and currently offer a limited repertoire of imaging contrasts and methods, they do allow for high-quality anatomical imaging (Deoni et al. 2022b) and have shown replication of developmental patterns observed at higher field strength (Deoni et al. 2021).
As much of the past work with low-field scanners has focused on clinical applications (e.g., identification of pediatric hydrocephalus (Sien et al. 2022), multiple sclerosis (Arnold et al. 2022), stroke (Yuen, et al. 2022), and other indications (Campbell-Washburn et al. 2019)), its utility in neuroscience research, such as associations between brain morphology and cognitive performance, remains unknown. Like remote cognitive assessments, the ability to reliably collect high-quality and information-rich MRI data at a participant’s home could bring new opportunities to the study of aging, cognitive decline, cognitive impairment, and dementia. Remote MRI would allow the inclusion of participants with mobility challenges or who lack transportation options, those who live long distances away from research centers and outside traditional recruitment areas, and those with competing family, work, or other time commitments. The reduced expense of low-field strength MRI may further allow for increased study population size, improving statistical power and generalizability of study findings.
To this end, in this study we sought to determine the feasibility of combining remote cognitive assessment via MindCrowd with mobile low field MRI in a modified Ford Transit cargo “scan van” equipped with a 64mT MRI scanner (Deoni et al. 2022c) to (1) Determine the feasibility of collecting reliable remote MRI and cognitive data in adults and elderly individuals; and (2) Replicate previously reported associations between regional brain volumes and cognitive performance with an established cognitive assessment, PAL, with substantial normative data against which to compare.
Methods
This study was performed in accordance with ethics approval and oversight by WCG IRB and the Rhode Island Hospital Institutional Review Board. All volunteers provided informed consent for both the MRI and neurocognitive assessment components.
Participants
A total of 75 individuals (42 female, 56%) between 18 and 94 years of age were recruited to participate in this study. Community recruitment was performed using print advertisements throughout the Providence area and to older members of families involved in other ongoing studies at our lab. Word-of-mouth quickly became the primary recruitment mechanism, with 47 participants learning about the study from a friend who was already enrolled. Six participants enrolled “at their doorstep” after noticing the van on their street and inquiring about the study. In total, recruitment and data collection required three weeks to reach a study population size that would be sensitive to medium effect sizes (r ~ 0.35) for correlations between imaging and cognitive measures.
Of the 75 recruited, 67 (39 female) completed the web-based cognitive assessments within 1 week of scanning and had high-quality MRI. 3 individuals did not complete the MindCrowd assessments in a timely fashion and 5 had poor quality or incomplete MRI (i.e., they were positioned too low in the coil). Included participants completed all 36 MindCrowd trials (described in more detail below), suggesting they understood the task. Participant demographics are provided in Table 1. The mean age of the final study population was 54.2 ± 19.7 years. In addition to providing age and biological sex, participants were also asked to indicate their education attainment on a 5-item scale (1 = Some High School, 2 = High School Diploma, 3 = Some College, 4 = College Degree, and 5 = Graduate Degree).
Recruitment, MRI scanning, and online assessment collection for this study were performed over a 3 week span. Scanning was performed either at the participant’s home or at a community center ‘pop-up’ event. Total imaging time, including set-up, was ~ 20 min per subject. Set-up time was slightly longer for individuals who required help into the van and onto the scanner bed. At the pop-up events, 2–3 scans were performed per hour with 8–12 individuals consented and scanned over a ~ 6 h afternoon.
Cognitive assessments
At enrollment and in follow-up emails after the study visit, participants were provided with login details and instructions to perform the PAL memory test on MindCrowd (mindcrowd.org). For participants without personal internet access, a MacBook Pro laptop or cellular internet-connected iPad tablet was made available for them. The PAL task is a type of episodic memory and learning task that requires a set of associations (e.g., a pair of words) be learned over several trials. The objective is to reduce errors with each trial. Individuals with higher cognitive functioning have increased PAL scores (i.e., they learn and remember the set of associations quicker).
As part of the registration process, participants were invited to provide general demographic details (age, biological sex, education attainment, spoken and written languages, country of residence, and zip code), as well as more specific sociodemographic, health history, and lifestyle information (e.g., race, ethnicity, handedness, number of daily prescription medications, as well as identifying if they have a first-degree family history of dementia, suffer or have suffered seizures, dizzy spells, loss of consciousness for more than 10 min, if they smoke, and if they have high blood pressure, diabetes, heart disease, cancer, stroke, alcohol/drug abuse, brain disease and/or memory problems).
For the online implementation of the PAL task, participants are first provided with an initial practice/example phase in which they are shown 3 word-pairs (e.g., apple | green, swim | suit, water | grass) for 2 s each. Then they are presented with the first word of each pair and are asked to use their keyboard to type (i.e., recall) the missing paired word. After the initial practice session, participants are then presented with 12 word-pairs with each pair presented for 2 s. The bank of word-pairs includes pairs with differing levels of difficulty (e.g., apple-pen, black-night), and does not include the pairs used for the practice session. Immediately following the presentation of pairs, participants are presented with the first word of each pair and are asked to use their keyboard to type (i.e., recall) the missing paired word.
This learning-recall procedure is repeated for two additional trials (3 trials in total). Word-pairs are presented in different random orders during each learning and each recall phase. The same word pairs and order of presentation is used for all participants. The dependent variable/criterion used in this study was the total number of correct word pairs entered across the 3 trials A perfect score is 36, with all 12 word-pairs remembered correctly on all 3 trials. Past work has shown that PAL scores are reduced in individuals with very early Alzheimer’s disease as well in normative ‘healthy’ aging (Baker et al. 2019; Lowndes et al. 2008).
Neuroimaging
MRI was performed on a commercial 64mT Hyperfine (Guilford, CT) Swoop system installed in a purpose-modified Ford Transit “scan van” (Sien et al. 2022) at each participant’s residence or nearby location (e.g., library or grocery store), Fig. 1. The Swoop system is the first commercially available and FDA-certified low-field MRI system for human imaging. Available since 2018, the system provides 4 main acquisition sequences: T1-weighted inversion-prepared (IR-) Fast Spin Echo (FSE), T2-weighted FSE, T2-weight Fluid Attenuated Inversion Recovery (FLAIR), and single direction diffusion-weighted FSE.
Imaging for this study included a series of anisotropic resolution T2-weighted 3D FSE images acquired in each of the three orthogonal orientations (axial, sagittal, and coronal) with an in-plane spatial resolution of 1.5 × 1.5 mm and a slice thickness of 5 mm. Specific imaging parameters in each direction are provided in Table 2. Unlike conventional 1.5 T, 3 T or other commercial MRI systems, the Swoop does not currently allow easy manipulation of imaging parameters, acquisition timings (echo, repetition, or inversion times), field of view, spatial resolution, or slice thickness.
While T1-weighted contrast is the standard for anatomical studies at 1.5 and 3 T, we have found the reduced T1 at 64 mT coupled with the implemented acquisition parameters reduces the quality of the T1-weighted images (e.g., Fig. 2) and that the T2-FSE images provide improved and more consistent tissue contrast across the age span (Deoni et al. 2021; Deoni et al. 2022c). Thus, T2-FSE images were used for this study.
As part of the pre-scan routine, a main magnetic field (B0) field map is acquired for shimming and gradient compensation. Electromagnetic interference (EMI) is also actively measured by three EMI detectors on the scanner and used for retrospective artifact removal similar to Srinivas et al. (2022). While temperature was not monitored throughout each scan, all scanning was performed within the temperature thresholds of the scanner (15–30 C) both to ensure consistency across scans as well as for participant comfort.
Overall, the scanning process was well-tolerated by participants and their families. Family members were able to be in the van during scanning or could wait outside watching through the van’s sliding door (Fig. 1). Following imaging, participants were asked about their experience, including if they were comfortable throughout the exam, if they experienced claustrophobia, and if they would recommend the study to others. If they had received a 1.5 or 3 T scan as part of a past research study or clinical exam, they were also asked if they rated this experience as more or less positive, and which they would prefer. While 8 participants complained about claustrophobia, participants overwhelmingly (100% of 43 who had had a past MRI scan) preferred being imaged at their home on the low-field system.
All imaging was performed by qualified research assistants who had received training from Hyperfine on use and safety monitoring of the system, and who have 2–3 years of scanning experience on commercial 3 T systems. The Swoop console is tablet-based and requires minimal training to use as the system automatically positions the field of view and acquisition protocols are not user-changeable.
MRI data processing
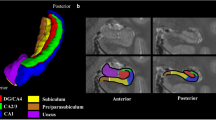
For image processing, a super-resolution reconstruction approach (Deoni et al. 2022b) was used to combine the orthogonal images into a single (1.5 × 1.5 × 1.5) mm3 image. A general study template was constructed using the ANTs 2.2 (Avants et al. 2011), antsMultivariateTemplateConstruction2.sh script using data from 26 study participants selected uniformly across the age range (Fig. 3, first and second column). The non-linear transformation matrix between this template and MNI space was calculated. Using this transformation, the MNI structural atlas (Avants et al. 2011; Rapp et al. 2013) was aligned to the study template to provide initial estimates of white matter (WM), gray matter (GM), and cerebral spinal fluid (CSF) masks. These masks were then used as initial priors for the ANTs Atropos tissue segmentation approach to refine the WM, GM, and CSF masks (Fig. 3, third column). Masks of deep brain structures (right and left hemisphere hippocampus, amygdala, caudate nucleus, thalamus, globus palliudus, and putamen) were also developed by transforming the Oxford-Harvard subcortical atlas provided as part of FSL (Srinivas et al. 2022) onto the study template (Fig. 3, fourth column).
Developed study template and regional tissue and sub-cortical structural masks. A custom study template was created using 26 study participants chosen uniformly cross the age range from 20 to 94 years of age and evenly split by male and female. White matter (green), Gray matter (blue) and CSF (red) masks were developed using the MNI structural atlas as priors and refined using ANTS Atropos. Subcortical deep brain masks were also constructed using the Oxford-Harvard subcortical atlas with Thalamus (yellow with red outline), Putamen (light blue), Globus Pallidus (dark blue), Caudate nucleus (purple with blue outline), Hippocampus (green) and Amygdala (white with pink outline)
Once the study template and tissue masks were created, each study participant’s SR anatomical image was aligned using ANTs, and the non-linear transformations and the corresponding Jacobean matrices were calculated and saved.
Correlation analysis
Following data collection, an initial exploratory voxel-based analysis was performed to identify potential associations between the collected cognitive measures (word-pair association score and reaction time) and brain gray matter density. Following non-linear alignment to the study template, correction for the effects of the warping on the voxel-wise density measures, and subtle blurring with a 4 mm Gaussian kernel, a general linear model was fit at each voxel that modeled PAL score (total correct word-pairs) as a function of local gray matter density (GM), subject age, and biological sex, i.e.,
Recognizing prior demonstrated associations between education level and metrics of brain morphometry in healthy and cognitively impaired individuals (Rapp et al. 2013; Cox et al. 2016), we extended our exploratory analyses to include reported education level as an additional model variable,
Education was scored on a 5-point scale ranging from 1 (high school diploma) to 5 (graduate or professional degree).
Analysis was performed using the Randomise tool of the FMRIB Software Library (FSL) (Jenkinson, et al. 2012). Threshold-free cluster enhancement (TFCE) (Smith and Nichols 2009) was used to control for the multiple voxel-wise comparisons.
Based on the outcomes of our exploratory analyses, hypothesis-based analysis was performed in which we examined associations between deep brain gray matter structure volumes (including right and left hemisphere hippocampus, amygdala, caudate nucleus, thalamus, globus palliudus, and putamen) and PAL scores. To calculate the deep gray matter structure volumes, the Oxford-Harvard subcortical atlas provided as part of FSL and registered to MNI space (Frazier et al. 2005) was aligned to each participant’s image using the inverse of their individual- > study template transformations. The aligned structure masks were then thresholded at 0.95 and the volumes of the inscribed regions were calculated.
In addition to sub-cortical gray matter volumes, we also used this atlas approach to examine the associations between total brain white matter (WM), gray matter (GM), and cerebral spinal fluid (CSF) volume and PAL scores. For these tissue volumes, the MNI structural atlas was used as the reference (Mazziotta et al. 2001; Haegelen et al. 2013). Examples of individual image quality and tissue mask alignment is shown in Fig. 4 for a young and older adult.
We hypothesized that brain regions involved in memory and learning networks, i.e., hippocampus, amygdala, caudate nucleus, and thalamus would be significantly associated with word-pair scores (Mori et al. 1997; Mizuno et al. 2000; Gould et al. 2005; Yoon et al. 2012; Pergola et al. 2013; Bauer et al. 2015; Barnett et al. 2016). This hypothesis was tested using a series of linear models that included PAL score and structure volume, as well as subject age, biological sex, and total brain tissue volume (the summation of white and gray matter volumes),
The Holm-Bonferroni approach was used to account for the 15 independent tests.
A simple model omitting the Structural Volume term was also fit to the data, allowing us to determine the additional variance explained by this variable.
Results
As a first check of data consistency, we plotted the total number of correct word pairs against subject age (Fig. 5), showing a decrease in performance as a function of age in agreement with past findings showing a decrease of 1–2 word-pairs per decade (Talboom, et al. 2019; Lewis et al. 2021).
To examine the MRI data, we plotted total brain white and gray matter volume percentage versus age (Fig. 6), observing the expected quadratic and linear trends for white and gray matter, respectively.
Figure 7 contains the results of our exploratory voxel-based analyses, highlighting brain areas with significant (p < 0.05 FWE) associations between gray matter density and PAL test scores. Modeling the association between local gray matter density and PAL performance, controlling for participant age, biological sex, and education attainment, we found significant associations predominately in left hemisphere regions, including hippocampus, parahippocampal gyrus, inferior temporal gyrus, thalamus, putamen, frontal pole and orbital cortex, caudate, and Broca’s area. In addition, right hemisphere precentral gyrus was also identified. These results did not substantively differ from the model that only included age and biological sex as additional variables of non-interest.
Exploratory voxel-based morphometry analysis examining associations between gray matter density and PAL score, controlling for subject age, biological sex, and education attainment. Highlighted regions denote significant associations (corrected for multiple comparisons using threshold-free cluster enhancement)
Table 3 contains the results of the linear modeling of regional subcortical gray matter volumes and PAL test scores, with representative plots of predicted vs. actual test scores as a function of regional volume, which are shown in Figs. 8, 9.
Results of the regional analyses build on the exploratory outcomes, showing hippocampus, amygdala, caudate, and thalamic volumes, as well as whole-brain white and gray matter, were significant predictors of PAL performance after correction for multiple comparisons (Table 3). In addition, right and left hemisphere putamen showed a trend toward significance. In each of these cases, the addition of the regional volume measure explained an additional 10 to 20% of the total variance in PAL performance. These numerical results are graphically represented in Figs. 8, 9, which show (Fig. 6) the measured PAL scores for each individual and predicted PAL by the linear model based on regional volumes as a function of age. The relationship between the measured and predicted PAL scores is shown in Fig. 9. As expected, we observe stronger correlations for brain regions where regional volume was a significant predictor of performance (e.g., white and gray matter vs. CSF volume).
Discussion
Here we have presented the first preliminary reports examining the practical use of a van-based portable low-field MRI scanner and web-based cognitive assessment to investigate changing total and regional brain volumes associated with cognitive performance. Results agree with prior reported outcomes, replicating the established general trends of white and gray matter change with age and identifying significant brain volume associations with memory performance in previously reported subcortical gray matter volumes. From our exploratory analyses, we found significant positive associations between improved PAL performance and increased local gray matter density in left hemisphere deep brain regions (hippocampus, thalamus, caudate, and putamen) as well as the left parahippocampal gyrus, frontal pole, and Broca’s area (Brodmann area 44), and right precentral gyrus (Brodmann area 4). These regions have known functional associations with language processing, memory, and learning. While the precentral gyrus is not directly related to these neurological functions, it is involved in movement and may reflect individual differences in tablet use or ability during the MindCrowd assessment. These results were confirmed in a follow-up regional analysis, which identified significant brain-PAL associations in the bilateral hippocampus, amygdala, thalamus, and caudate, controlling for subject age, sex, educational attainment, and total brain volume. Although the putamen associations did not survive FWE correction (with corrected p values of approximately ≃ 0.05) they do show a strong trend toward significance.
These results carry important implications for future opportunities and directions in neuroscience research. The combination of mobile at-home neuroimaging and web-based remote assessment presents an important new opportunity to engage individuals from socially, economically, and educationally disadvantaged communities that are often under-represented in clinical and public health research (Yoon et al. 2012; Pergola et al. 2013). Moreover, some clinical and pre-clinical populations (e.g., individuals in their 30 or 40 s with a family history of Alzheimer’s disease but without personal memory or cognitive complaints) are often difficult to recruit and/or retain in long-term longitudinal trials and studies because of family and work time commitments. By bringing the scanner to them at home or work, and allowing cognitive assessments to be performed on their time, involving these important cohorts may be less challenging. Though our study sample was predominately from higher educational (~ 34% reported a college or post-graduate degree) and non-Latino/Hispanic Caucasian (86%) backgrounds, there is nothing that fundamentally limits our approach from reaching broader individuals and communities.
While memory changes are experienced by most, but not all, individuals as they age, worsened associative memory performance can also be indicative of emerging cognitive impairment and dementia (Andriuta et al. 2019; Barnett et al. 2016; Fowler et al. 2002; Haynes et al. 2017). Neuroanatomical correlates of reduced performance on various episodic memory tests, including associative memory, include reduced total gray matter volume and regional reductions in the hippocampus, thalamus, and putamen. Alongside these functionally related changes, progressive volume loss in the entorhinal cortex, frontal lobe, and temporoparietal cortical areas have been implicated in mild cognitive impairment (MCI) (Apostolova and Thompson 2008). Increased rates of volume loss in these regions are potentially predictive of progression from MCI to Alzheimer’s disease (Jack et al. 2000).
A recent report on the state of study needs in aging research (Watson et al. 2014) has estimated that nearly 100,000 participants will need to be recruited into the existing set of US-based observational and clinical trials for prospective preventative or therapeutic AD treatments. The authors further estimate that achieving this level of enrollment will require screening upwards of 1 million potential participants and their families. As with other scientific and clinical health research, research in aging and AD has suffered from under-representation of individuals from racial and ethnic minorities, and economically disadvantaged communities (Karlawish et al. 2008)—despite these groups having a higher potential risk for dementia (Tang et al. 2001). A further missing gap is individuals who are free of clinical symptoms but at risk for AD, since initiating pathology may appear 2 or 3 decades before overt memory loss or other symptoms become apparent (Beason-Held et al. 2013). This necessitates recruitment and longitudinal retention of 30 to 50 year-old individuals. These individuals, however, often have busy family, work, and social schedules, which inhibits participation in research studies. To address these challenges, Watson et al. (Watson et al. 2014) highlight the need to consider and accommodate the location and time needs of participants and their families (or study partners) by conveniently locating study sites or, ideally, performing study visits at the participant’s home.
The results presented here highlight the potential to perform participant screening, enrollment, and at-home study visits, even in the context of neuroimaging studies. While we have presented MindCrowd as an effective tool for remote cognitive assessment, it can also be envisaged and utilized as a screening tool, allowing the identification of individuals willing to participate in research studies, and with important clinical and/or sociodemographic phenotypes. Beyond cognitive and health and family history information, participants can also provide biological samples from at-home collections (e.g., saliva samples for genomic analyses) allowing genetic phenotypes to also be screened. We purposefully designed our Scan-Van on a 2021 Ford Transit Van base (high roof and extended length 2500 model with a 9500lbs gross vehicle weight rating) to achieve three functional aims: 1. Ability to travel on local and dirt roads to allow access to rural communities and not rely on truck or high weight capacity routes. 2. To be driven by anyone with a regular driver’s license (i.e., not require a commercial CDL license). And 3. Have access to a large national network of maintenance and repair facilities with ready access to parts and service. In addition, we also designed the system to use an EGO Power + 3000 W portable power station that provides more than 6 h of continuous scanning from 4 rechargeable (with additional batteries able to be hot swapped to allow longer scanning), and for the ability to load and unload the scanner for imaging in or outside the vehicle.
While 5 scans were rejected due to poor subject positioning in the coil (all collected on the same day of scanning), this error was corrected and no further scans were rejected for this or other quality reasons (e.g., motion artifacts, noise, poor contrast, etc.). As all scans for this study were performed in the van, which required participants to be mobile enough to walk up 2 steps (~ 18 inches) into the van and then onto a 30″ high massage bed. For larger scale studies, the scanner can be removed from the van to accommodate participants with mobility challenges and unable to get into the van. To allow the scanner to be used in the fall and winter months (in New England) and avoid participant discomfort or operating outside of the scanner’s recommended range (5–30 C), a heating system was built into the van that could be complemented with a portable electric heater (also run from the portable battery station). In the summer months, operating with the rear doors open and an oscillating fan provides sufficient comfort for the short scan duration without impacting scan quality. In cases of extreme heat, a roof-mounted air-conditioning unit can also be used.
While most prior studies in aging and AD, including the original ADNI protocol (Jack et al. 2008), focused on structural and morphology changes, more recent investigations have included assessment of tissue micro-structure (diffusion tensor imaging, DTI), cerebral perfusion, and structural and functional connectivity. Currently, the Hyperfine system is capable of four structural image contrasts (T1, T2, T2-FLAIR, and single-axis DWI). As more research groups gain access to these portable and lower field strength systems, it is likely we will see steady improvements in acquisition techniques, including DTI, relaxometry, and potentially perfusion imaging.
In this work, we used T2-weighted data as opposed to the more conventional T1-weighted acquisitions that are the mainstay of 1.5 and 3 T adult neuroimaging. This decision was based on the current limitations of the Hyperfine system (noted above and in the Methods) and our ongoing experience with Hyperfine T1 and T2 data quality (e.g., Fig. 2). It is expected that as additional improvements are made to the acquisition techniques, specifically the development of rapid steady-state based acquisitions (e.g., spoiled or balanced steady-state gradient echo sequences (Scheffler and Hennig 2003)) improved T1 contrast will be possible given both the shortening and increased dispersion of tissue T1 characteristics at lower field strength. However, to inform on potential biases introduced by the use of T2 data, we compared total white matter and regional putamen, amygdala, and hippocampus volumes collected from low-field T2 and high-field T1-weighted MP-RAGE data in 15 individuals (11 female, 25 ± 17 years of age) not drawn from the current study sample. MP-RAGE data was collected using the ADNI protocol (Jack et al. 2008). Ad-hoc results, shown in Fig. 10, derived using the same atlas-based approach for both image contrasts, showed agreement between the measures without significant non-zero bias.
Blad-Altman plots showing the differences in whole-brain white and regional left-hemisphere putamen, amygdala, and hippocampus volumes measured from T2-weighted low-field data and conventional T1-weighted 3 T data in 15 adult individuals. No significant non-zero bias is observed between the sets of measures
A potential source of variability that was not directly accounted for here is the type and size of the device used for the MindCrowd assessment. Participants were provided either a 13″ MacBook Pro or a 10.9″ iPad Air to complete the online cognitive assessments at the van. Participants who completed the assessments at home may have used a differently sized laptop/desktop computer, tablet, or mobile phone. These differences in screen size and keyboard size and type (physical vs. virtual touch screen) may have impacted PAL performance and added additional variability to PAL performance. While further investigation is needed to understand this potential variability (and accurately account for it), we do not believe it will substantively alter the presented results. For the PAL assessment, individuals are allowed 10 s to begin their response and are allowed ample completion time before the system moves on to the next word-pair. Thus, challenges with a smaller or unfamiliar keyboard should not significantly reduce their total score.
A general challenge with online unassisted assessment is knowing when low scores are accurate reflections of an individual’s ability or are the result of them not paying attention during the assessment or not understanding/following instructions. For example, in our cohort, 4 individuals under 45 years of age reported PAL scores of less than 10. These scores are more than 3 standard deviations below the expected mean for healthy individuals of their age (Talboom et al. 2021). As an ad-hoc analysis, we repeated our regional volume analyses excluding these individuals as outliers. The results of this analysis did not differ from those presented in Table 3, with the exception that right putamen volume remained a significant predictor after Holm-Bonferroni correction for the multiple tests.
In summary, the work described here demonstrates the feasibility of coupling mobile neuroimaging and web-based cognitive assessments. With this approach, we replicate known effects of aging on associative memory performance as well as highlight neuroimaging-based changes in learning and memory-based regions of the brain with performance. We propose that the combination of a mobile neuroimaging laboratory with on-demand web-based cognitive assessment has significant potential for the future study of many types of disadvantaged and understudied populations, including race-ethnic, socioeconomic, time-constrained, and geographically distanced groups. More work is necessary to refine our approach and appropriately tailor recruiting and retention practices to ensure success with such groups, however, we propose that our work demonstrates the significant potential for these efforts.
Conclusions
In this study we sought to (1) Determine the feasibility of collecting remote MRI and cognitive data in adults and elderly individuals; and (2) Replicate previously reported population-based associations between regional brain volumes and cognitive performance with an established cognitive assessment, PAL. Overall, this initial report of at-home MRI has suggested that the collection of MRI data on a portable low-field MRI system is possible and offers time efficiency, convenience, and accessibility to participants who might otherwise not be able to participate. Indeed, individuals in our study who have had a prior MRI at a clinical or research setting all preferred the at-home approach as well as the open nature of the low-field system. Collected data and results align with past reports of brain-memory function in aging adults, highlighting associations between the hippocampus, thalamus, and caudate nucleus and PAL performance. While further work is required to firmly establish the utility of low-field MRI in neuroscience and neurocognitive research, this preliminary work provides a foundation and support for this potential new direction in MRI research.
Data availability
All de-identified imaging and neurocognitive data collected as part of this study are available upon request of the corresponding author.
References
Alcohol Research: Current Reviews Editorial S (2018) NIH’s adolescent brain cognitive development (ABCD) Study. Alcohol Res 39(1):97
Alfaro-Almagro F et al (2018) Image processing and quality control for the first 10,000 brain imaging datasets from UK biobank. Neuroimage 166:400–424
Aljondi R et al (2019) A decade of changes in brain volume and cognition. Brain Imaging Behav 13(2):554–563
Ambrose SE, VanDam M, Moeller MP (2014) Linguistic input, electronic media, and communication outcomes of toddlers with hearing loss. Ear Hear 35(2):139–147
Andriuta D et al (2019) Is Reaction time slowing an early sign of Alzheimer’s disease? A Meta-Analysis Dement Geriatr Cogn Disord 47(4–6):281–288
Anstey KJ et al (2007) Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia 45(8):1911–1920
Apostolova LG, Thompson PM (2008) Mapping progressive brain structural changes in early Alzheimer’s disease and mild cognitive impairment. Neuropsychologia 46(6):1597–1612
Armstrong NM et al (2020) Associations between cognitive and brain volume changes in cognitively normal older adults. Neuroimage 223:117289
Arnold TC et al (2022) Sensitivity of portable low-field magnetic resonance imaging for multiple sclerosis lesions. Neuroimage Clin 35:103101
Avants BB et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54(3):2033–2044
Baker JE et al (2019) Visual paired associate learning deficits associated with elevated beta-amyloid in cognitively normal older adults. Neuropsychology 33(7):964–974
Barkovich AJ et al (1988) Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 166(1 Pt 1):173–80
Barnett JH et al (2016) The paired associates learning (PAL) test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia research. Curr Top Behav Neurosci 28:449–474
Batista S et al (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259(1):139–146
Bauer E et al (2015) The significance of caudate volume for age-related associative memory decline. Brain Res 1622:137–148
Beason-Held LL et al (2013) Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 33(46):18008–18014
Bethlehem RAI et al (2022) Brain charts for the human lifespan. Nature 604(7906):525–533
Bookheimer SY et al (2019) The lifespan human connectome project in aging: an overview. Neuroimage 185:335–348
Campbell-Washburn AE et al (2019) Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 293(2):384–393
Caskey M et al (2014) Adult talk in the NICU with preterm infants and developmental outcomes. Pediatrics 133(3):e578–e584
Cox SR et al (2016) Associations between education and brain structure at age 73 years, adjusted for age 11 IQ. Neurology 87(17):1820–1826
Cox SR et al (2019) Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76:101376
Deoni SCL et al (2021) Accessible pediatric neuroimaging using a low field strength MRI scanner. Neuroimage 238:118273
Deoni SCL et al (2022a) Remote and at-home data collection: considerations for the NIH HEALthy brain and cognitive development (HBCD) study. Dev Cogn Neurosci 54:101059
Deoni SCL et al (2022b) Simultaneous high-resolution T2 -weighted imaging and quantitative T2 mapping at low magnetic field strengths using a multiple TE and multi-orientation acquisition approach. Magn Reson Med 88(3):1273–1281
Deoni SCL et al (2022c) Development of a mobile low-field MRI scanner. Sci Rep 12(1):5690
Dumurgier J et al (2012) MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage 60(2):871–878
Duncan GJ et al (2007) School readiness and later achievement. Dev Psychol 43(6):1428–1446
Dykstra JR et al (2013) Using the language environment analysis (LENA) system in preschool classrooms with children with autism spectrum disorders. Autism 17(5):582–594
Elam JS et al (2021) The human connectome project: a retrospective. Neuroimage 244:118543
Elderkin-Thompson V et al (2008) Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology 22(5):626–637
Elderkin-Thompson V et al (2009) Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry 24(5):459–468
Ferjan Ramirez N, Lytle SR, Kuhl PK (2020) Parent coaching increases conversational turns and advances infant language development. Proc Natl Acad Sci U S A 117(7):3484–3491
Fields RD (2015) A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16(12):756–767
Fling BW et al (2011) Age differences in callosal contributions to cognitive processes. Neuropsychologia 49(9):2564–2569
Forget-Dubois N et al (2009) Early child language mediates the relation between home environment and school readiness. Child Dev 80(3):736–749
Fowler KS et al (2002) Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc 8(1):58–71
Fox NC et al (1996) Presymptomatic hippocampal atrophy in Alzheimer’s disease. A Longitudinal MRI Study Brain 119(Pt 6):2001–2007
Frazier JA et al (2005) Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 162(7):1256–1265
Geddes MR et al (2020) Remote cognitive and behavioral assessment: report of the Alzheimer society of Canada task force on dementia care best practices for COVID-19. Alzheimers Dement (amst) 12(1):e12111
Gilkerson J et al (2015) Evaluating language environment analysis system performance for Chinese: a pilot study in Shanghai. J Speech Lang Hear Res 58(2):445–452
Gilkerson J et al (2018) Language experience in the second year of life and language outcomes in late childhood. Pediatrics 142(4)
Goldfarb MG, Brown DR (2022) Diversifying participation: The rarity of reporting racial demographics in neuroimaging research. Neuroimage 254:119122
Goodro M et al (2012) Age effect on subcortical structures in healthy adults. Psychiatry Res 203(1):38–45
Gould RL et al (2005) Functional neuroanatomy of successful paired associate learning in Alzheimer’s disease. Am J Psychiatry 162(11):2049–2060
Haegelen C et al (2013) Automated segmentation of basal ganglia and deep brain structures in MRI of Parkinson’s disease. Int J Comput Assist Radiol Surg 8(1):99–110
Haynes BI, Bauermeister S, Bunce D (2017) A systematic review of longitudinal associations between reaction time intraindividual variability and age-related cognitive decline or impairment, dementia, and mortality. J Int Neuropsychol Soc 23(5):431–445
Hill JR et al (2021) Going remote-demonstration and evaluation of remote technology delivery and usability assessment with older adults: survey study. JMIR Mhealth Uhealth 9(3):e26702
Hirsh-Pasek K et al (2015) The contribution of early communication quality to low-income children’s language success. Psychol Sci 26(7):1071–1083
Hooyman A et al (2021) Remote, unsupervised functional motor task evaluation in older adults across the United States using the mindcrowd electronic cohort. Dev Neuropsychol 46(6):435–446
Huentelman MJ et al (2020) Reinventing neuroaging research in the digital age. Trends Neurosci 43(1):17–23
Jack CR Jr et al (2000) Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55(4):484–489
Jack CR Jr et al (2008) The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging 27(4):685–691
Jackson JD et al (2012) White matter integrity and reaction time intraindividual variability in healthy aging and early-stage Alzheimer disease. Neuropsychologia 50(3):357–366
Jenkinson M et al (2012) Fsl. Neuroimage 62(2):782–90
Karlawish J et al (2008) How redesigning AD clinical trials might increase study partners’ willingness to participate. Neurology 71(23):1883–1888
King LS et al (2020) Naturalistic language input is associated with resting-state functional connectivity in infancy. J Neurosci
Konkel L (2015) Racial and ethnic disparities in research studies: the challenge of creating more diverse cohorts. Environ Health Perspect 123(12):A297-302
Lenroot RK et al (2007) Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36(4):1065–1073
Lewis CR et al (2021) Smoking is associated with impaired verbal learning and memory performance in women more than men. Sci Rep 11(1):10248
Long X et al (2012) Healthy aging: an automatic analysis of global and regional morphological alterations of human brain. Acad Radiol 19(7):785–793
Lowndes GJ et al (2008) Recall and recognition measures of paired associate learning in healthy aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 15(4):506–522
Lurie LA et al (2021) Mechanisms linking socioeconomic status and academic achievement in early childhood: Cognitive stimulation and language. Cogn Dev 58
Masek LR et al (2021) Beyond talk: contributions of quantity and quality of communication to language success across socioeconomic strata. Infancy 26(1):123–147
Mazziotta J et al (2001) A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 356(1412):1293–1322
McMahon E, Wintermark P, Lahav A (2012) Auditory brain development in premature infants: the importance of early experience. Ann N Y Acad Sci 1252:17–24
Mizuno K et al (2000) Medial temporal atrophy and memory impairment in early stage of Alzheimer’s disease: an MRI volumetric and memory assessment study. J Neurol Sci 173(1):18–24
Mori E et al (1997) Medial temporal structures relate to memory impairment in Alzheimer’s disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry 63(2):214–221
Narvacan K et al (2017) Evolution of deep gray matter volume across the human lifespan. Hum Brain Mapp 38(8):3771–3790
Pergola G et al (2013) The role of the thalamic nuclei in recognition memory accompanied by recall during encoding and retrieval: an fMRI study. Neuroimage 74:195–208
Phillips M et al (2013) Intra-individual reaction time variability in mild cognitive impairment and Alzheimer’s disease: gender, processing load and speed factors. PLoS ONE 8(6):e65712
Rapp SR et al (2013) Educational attainment, MRI changes, and cognitive function in older postmenopausal women from the women’s health initiative memory study. Int J Psychiatry Med 46(2):121–143
Rodrigo A et al (2021) Identification of undiagnosed dementia cases using a web-based pre-screening tool: the MOPEAD project. Alzheimers Dement 17(8):1307–1316
Romano E et al (2010) School readiness and later achievement: replication and extension using a nationwide Canadian survey. Dev Psychol 46(5):995–1007
Romeo RR et al (2018a) Beyond the 30-million-word gap: children’s conversational exposure is associated with language-related brain function. Psychol Sci 29(5):700–710
Romeo RR et al (2018b) Language exposure relates to structural neural connectivity in childhood. J Neurosci 38(36):7870–7877
Rowe ML (2012) A longitudinal investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Dev 83(5):1762–1774
Scheffler K, Hennig J (2003) Is TrueFISP a gradient-echo or a spin-echo sequence? Magn Reson Med 49(2):395–397
Shi F et al (2009) Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus 19(11):1055–1064
Sien ME et al (2022) Feasibility of and experience using a portable MRI scanner in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1):83–98
Srinivas SA et al (2022) External dynamic interference estimation and removal (EDITER) for low field MRI. Magn Reson Med 87(2):614–628
Suskind DL et al (2016) A parent-directed language intervention for children of low socioeconomic status: a randomized controlled pilot study. J Child Lang 43(2):366–406
Taki Y et al (2011) Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS ONE 6(7):e22734
Talboom JS et al (2019) Family history of Alzheimer’s disease alters cognition and is modified by medical and genetic factors. Elife 8:e46179
Talboom JS et al (2021) Two separate, large cohorts reveal potential modifiers of age-associated variation in visual reaction time performance. NPJ Aging Mech Dis 7(1):14
Tang MX et al (2001) Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 56(1):49–56
Terribilli D et al (2011) Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol Aging 32(2):354–368
Thompson LI et al (2022) A highly feasible, reliable, and fully remote protocol for mobile app-based cognitive assessment in cognitively healthy older adults. Alzheimers Dement (amst) 14(1):e12283
Tsiakiri A et al (2022) Remote neuropsychological evaluation of older adults. Appl Neuropsychol Adult. https://doi.org/10.1080/23279095.2022.2074850
van der Knaap MS et al (1991) Myelination as an expression of the functional maturity of the brain. Dev Med Child Neurol 33(10):849–857
Van Der Werf YD et al (2001) Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Brain Res Cogn Brain Res 11(3):377–85
von Stumm S et al (2020) Preschool verbal and nonverbal ability mediate the association between socioeconomic status and school performance. Child Dev 91(3):705–714
Walhovd KB, Fjell AM (2007) White matter volume predicts reaction time instability. Neuropsychologia 45(10):2277–2284
Walker D et al (1994) Prediction of school outcomes based on early language production and socioeconomic factors. Child Dev 65(2 Spec No):606–21
Watson JL et al (2014) Obstacles and opportunities in Alzheimer’s clinical trial recruitment. Health Aff (millwood) 33(4):574–579
Webb AR et al (2015) Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci U S A 112(10):3152–3157
Weiner MW et al (2013) The Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement 9(5):e111–e194
Weisleder A, Fernald A (2013) Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychol Sci 24(11):2143–2152
Westlye LT et al (2010) Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20(9):2055–2068
Yoon J et al (2012) Hippocampus is required for paired associate memory with neither delay nor trial uniqueness. Learn Mem 19(1):1–8
Yuen MM et al (2022) Portable, low-field magnetic resonance imaging enables highly accessible and dynamic bedside evaluation of ischemic stroke. Sci Adv 8(16):eabm3952
Ziegler G et al (2012) Brain structural trajectories over the adult lifespan. Hum Brain Mapp 33(10):2377–2389
Zimmerman FJ et al (2009) Teaching by listening: the importance of adult-child conversations to language development. Pediatrics 124(1):342–349
Acknowledgements
The authors extend important thanks to all study volunteers and their families, as well as Neil Sharpe and the Brain Injury Association of Rhode Island.
Funding
National Institute on Drug Abuse (SCD R34DA050284). MindCrowd receives support from the Mueller Family Charitable Trust, the Flinn Foundation, the State of Arizona DHS in support of the Arizona Alzheimer’s Consortium, and the National Institutes of Health–National Institute on Aging (U19AG065169).
Author information
Authors and Affiliations
Contributions
SD: conceptualization, formal analysis, resources, writing, supervision, funding; MH: conceptualization, methodology, writing review, supervision, resources, funding; LR: methodology, writing review, supervision, resources, funding; JB: methodology, data collection, writing review; PB: methodology, data collection, writing review; RC-L: methodology, data collection, writing review; MDB: methodology, data collection, writing review; MJ: methodology, data collection, writing review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deoni, S.C.L., Burton, P., Beauchemin, J. et al. Neuroimaging and verbal memory assessment in healthy aging adults using a portable low-field MRI scanner and a web-based platform: results from a proof-of-concept population-based cross-section study. Brain Struct Funct 228, 493–509 (2023). https://doi.org/10.1007/s00429-022-02595-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02595-7