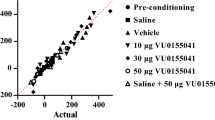
Abstract
Although the firing activity of dopamine (DA) neurons in the ventral tegmental area (VTA) and the behavioral response to morphine rewarding properties alter as opiate withdrawal, little is known about the dynamic changes in DA signal pathway from the VTA to the nucleus accumbens (NAc) during prolonged withdrawal, and whether the changes are indicative of vulnerability to relapse of drug abuse. Here we report that morphine spontaneously withdrawn (SW) rats are incapable of responding to small dose of morphine-induced conditioned place preference (CPP) from 24h-SW to 30d-SW, but recover response at 45d-SW. Interestingly, mesoaccumbens DA signaling, including the firing of DA neurons in the VTA, contents of DA and its metabolic ratio, and the membrane level of dopamine D1 receptor in the NAc elicited by morphine challenge, display a similar pattern of time-dependent changes during morphine withdrawal. Moreover, blockade of D1 receptor abolishes this behavioral transition. In addition, a strong correlation was found between % change in CPP score and membrane D1 receptor level induced by morphine challenge. These results indicate a time-dependent behavioral switch from tolerance to sensitization during the prolonged withdrawal, which could offer a window for therapeutic intervention via manipulations of D1 receptors.








Similar content being viewed by others
References
Acquas E, Di Chiara G (1992) Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem 58(5):1620–1625
Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22(4):413–421
Bardo MT, Rowlett JK, Harris MJ (1995) Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19(1):39–51
Bonci A, Williams JT (1997) Increased probability of GABA release during withdrawal from morphine. J Neurosci 17(2):796–803
Chartoff EH, Mague SD, Carlezon Jr WA (2003) Effect of dopamine D1 receptor agonists on precipitated opiate withdrawal-induced place aversions in rats. Society for Neuroscience Abstract Viewer and Itinerary Planner 2003: Abstract No. 110.119
Chartoff EH, Barhight MF, Mague SD, Sawyer AM, Carlezon WA (2009) Anatomically dissociable effects of dopamine D1 receptor agonists on reward and relief of withdrawal in morphine-dependent rats. Psychopharmacology 204(2):227–239
Chergui K, Suaudchagny MF, Gonon F (1994) Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat-brain in vivo. Neuroscience 62(3):641–645
Chiodo LA (1988) Dopamine-containing neurons in the mammalian central nervous system—electrophysiology and pharmacology. Neurosci Biobehav Rev 12(1):49–91
Chu NN, Zuo YF, Meng L, Lee DYW, Han JS, Cui CL (2007) Peripheral electrical stimulation reversed the cell size reduction and increased BDNF level in the ventral tegmental area in chronic morphine-treated rats. Brain Res 1182:90–98
Chu NN, Xia W, Yu P, Hu L, Zhang R, Cui CL (2008) Chronic morphine-induced neuronal morphological changes in the ventral tegmental area in rats are reversed by electroacupuncture treatment. Addict Biol 13(1):47–51
Di Chiara G (1999) Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol 375(1–3):13–30
Di Chiara G, North RA (1992) Neurobiology of opiate abuse. Trends Pharmacol Sci 13(5):185–193
Diana M, Pistis M, Muntoni A, Gessa G (1995) Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther 272(2):781–785
Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M (2010) Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci 30(1):218–229
Fields HL, Hjelmstad GO, Margolis EB, Nicola SM (2007) Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci 30:289–316
Georges F, Aston-Jones G (2002) Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22(12):5173–5187
Georges F, Le Moine C, Aston-Jones G (2006) No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci 26(21):5720–5726
Gonon FG (1988) Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic-neurons as studied by in vivo electrochemistry. Neuroscience 24(1):19–28
Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons.1. Identification and characterization. Neuroscience 10(2):301–315
Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y (2003) Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 23(3):742–747
Guyenet PG, Aghajanian GK (1978) Antidromic identification of dopaminergic and other output neurons of rat substantia nigra. Brain Res 150(1):69–84
Gysling K, Wang RY (1983) Morphine-induced activation of a10 dopamine neurons in the rat. Brain Res 277(1):119–127
Herz A (1998) Opioid reward mechanisms: a key role in drug abuse? Can J Physiol Pharmacol 76(3):252–258
Hu L, Chu NN, Sun LL, Zhang R, Han JS, Cui CL (2009) Electroacupuncture treatment reverses morphine-induced physiological changes in dopaminergic neurons within the ventral tegmental area. Addict Biol 14(4):431–437
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug-induced and stress-induced sensitization of motor-activity. Brain Res Rev 16(3):223–244
Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006) Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26(22):5894–5900
Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han M-H, Nestler EJ (2012) BDNF is a negative modulator of morphine action. Science 338(6103):124–128
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24(2):97–129
Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8(11):1442–1444
Laviolette SR, Nader K, van der Kooy D (2002) Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res 129(1–2):17–29
Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47:214–226
Ma YY, Chu NN, Guo CY, Han JS, Cui CL (2007) NR2B-containing NMDA receptor is required for morphine-but not stress-induced reinstatement. Exp Neurol 203(2):309–319
Ma YY, Meng L, Guo CY, Han JS, Lee DY, Cui CL (2009) Dose- and time-dependent, context-induced elevation of dopamine and its metabolites in the nucleus accumbens of morphine-induced CPP rats. Behav Brain Res 204(1):192–199
Manzanedo C, Aguilar MA, Rodriguez-Arias M, Minarro J (2005) Sensitization to the rewarding effects of morphine depends on dopamine. Neuroreport 16(2):201–205
Manzoni OJ, Williams JT (1999) Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci 19(15):6629–6636
Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M, Krishnan V, Kim S, Siuta MA, Galli A, Niswender KD, Appasani R, Horvath MC, Neve RL, Worley PF, Snyder SH, Hurd YL, Cheer JF, Han MH, Russo SJ, Nestler EJ (2011) Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 72(6):977–990
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, New York
Pothos E, Rada P, Mark GP, Hoebel BG (1991) Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res 566(1–2):348–350
Rossetti ZL, Hmaidan Y, Gessa GL (1992) Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol 221(2–3):227–234
Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ (2007) IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci 10(1):93–99
Schultz W (2002) Getting formal with dopamine and reward. Neuron 36(2):241–263
Shi XD, Wang GB, Ma YY, Ren W, Luo F, Cui CL, Han JS (2004) Repeated peripheral electrical stimulations suppress both morphine-induced CPP and reinstatement of extinguished CPP in rats: accelerated expression of PPE and PPD mRNA in NAc implicated. Brain Res Mol Brain Res 130(1–2):124–133
Shippenberg TS, Emmettoglesby MW, Ayesta FJ, Herz A (1988) Tolerance and selective cross-tolerance to the motivational effects of opioids. Psychopharmacology 96(1):110–115
Shippenberg TS, Bals-Kubik R, Herz A (1993) Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther 265(1):53–59
Shippenberg TS, Heidbreder C, Lefevour A (1996) Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur J Pharmacol 299(1–3):33–39
Shippenberg TS, LeFevour A, Thompson AC (1998) Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol 345(1):27–34
Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ (1996) Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA 93(20):11202–11207
Sterling P, Eyer J (1988) Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J (eds) Handbook of life stress, cognition and health, vol xxxiii. Wiley, Oxford, England, pp 629–649
Sun W (2011) Dopamine neurons in the ventral tegmental area: drug-induced synaptic plasticity and its role in relapse to drug-seeking behavior. Curr Drug Abuse Rev 4(4):270–285
Teismann P, Ferger B (2001) Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39(2):167–174
Tepper JM, Young SJ, Groves PM (1984) Autoreceptor-mediated changes in dopaminergic terminal excitability—effects of increases in impulse flow. Brain Res 309(2):309–316
Thiblin I, Finn A, Ross SB, Stenfors C (1999) Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br J Pharmacol 126(6):1301–1306
Ungless MA, Magill PJ, Bolam JP (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303(5666):2040–2042
Willins DL, Meltzer HY (1998) Serotonin 5-HT2C agonists selectively inhibit morphine-induced dopamine efflux in the nucleus accumbens. Brain Res 781(1–2):291–299
Acknowledgments
This project was supported by grants from the National Basic Research Program (2009CB522003) and the National Natural Science Foundation (31271163) of China to Cailian Cui. We thank Dr. Lu Lin of the National Institute on Drug Dependence, Peking University, Beijing 100191, China, for his helpful comments on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Sun, L. Hu and Y. Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, L., Hu, L., Li, Y. et al. Mesoaccumbens dopamine signaling alteration underlies behavioral transition from tolerance to sensitization to morphine rewarding properties during morphine withdrawal. Brain Struct Funct 219, 1755–1771 (2014). https://doi.org/10.1007/s00429-013-0599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0599-2