Abstract
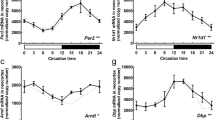
The circadian master clock of the mammalian brain resides in the suprachiasmatic nucleus (SCN) of the hypothalamus. At the molecular level, the clock of the SCN is driven by a transcriptional/posttranslational autoregulatory network with clock gene products as core elements. Recent investigations have shown the presence of peripheral clocks in extra-hypothalamic areas of the central nervous system. However, knowledge on the clock gene network in the cerebral cortex is limited. We here show that the mammalian clock genes Per1, Per2, Per3, Cry1, Cry2, Bmal1, Clock, Nr1d1 and Dbp are expressed in the rat neocortex. Among these, Per1, Per2, Per3, Cry1, Bmal1, Nr1d1 and Dbp were found to exhibit daily rhythms. The amplitude of circadian oscillation in neocortical clock gene expression was damped and the peak delayed as compared with the SCN. Lesions of the SCN revealed that rhythmic clock gene expression in the neocortex is dependent on the SCN. In situ hybridization and immunohistochemistry showed that products of the canonical clock gene Per2 are located in perikarya throughout all areas of the neocortex. These findings show that local circadian oscillators driven by the SCN reside within neurons of the neocortex.





Similar content being viewed by others
Abbreviations
- DD:
-
Dark–dark lighting regime (constant darkness)
- LD:
-
Light–dark lighting regime
- qPCR:
-
Quantitative real-time reverse-transcription PCR
- SCN:
-
Suprachiasmatic nucleus
- ZT:
-
Zeitgeber time
References
Abe H, Honma S, Namihira M, Tanahashi Y, Ikeda M, Honma K-i (1998) Circadian rhythm and light responsiveness of BMAL1 expression, a partner of mammalian clock gene Clock, in the suprachiasmatic nucleus of rats. Neurosci Lett 258:93–96
Abe H, Honma S, Namihira M, Masubuchi S, Ikeda M, Ebihara S, Honma K-i (2001) Clock gene expressions in the suprachiasmatic nucleus and other areas of the brain during rhythm splitting in CS mice. Mol Brain Res 87:92–99
Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD (2002) Circadian rhythms in isolated brain regions. J Neurosci 22:350–356
Abe H, Honma S, Ohtsu H, Honma K-i (2004) Circadian rhythms in behavior and clock gene expressions in the brain of mice lacking histidine decarboxylase. Mol Brain Res 124:178–187
Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR, Herzog ED (2005) Independent circadian oscillations of Period1 in specific brain areas in vivo and in vitro. J Neurosci 25:8620–8626
Albrecht U, Sun ZS, Eichele G, Lee CC (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91:1055–1064
Angeles-Castellanos M, Mendoza J, Escobar C (2007) Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 144:344–355
Antoch MP, Song E-J, Chang A-M, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS (1997) Functional identification of the mouse circadian clock gene by transgenic BAC rescue. Cell 89:655–667
Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S (2001) Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66:1133–1139
Aton SJ, Herzog ED (2005) Come together, right…now: synchronization of rhythms in a mammalian circadian clock. Neuron 48:531–534
Bass J, Takahashi JS (2010) Circadian integration of metabolism and energetics. Science 330:1349–1354
Buhr ED, Yoo S-H, Takahashi JS (2010) Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330:379–385
Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009–1017
Bunney JN, Potkin SG (2008) Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull 86:23–32
Coogan AN, Papachatzaki MM, Clemens C, Baird A, Donev RM, Joosten J, Zachariou V, Thome J (2011) Haloperidol alters circadian clock gene product expression in the mouse brain. World J Biol Psychiatry 12:638–644
DeFelipe J (1997) Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14:1–19
Druga R (2009) Neocortical inhibitory system. Folia Biol (Praha) 55:201–217
Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S (2006) Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 147:3769–3776
Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280:1564–1569
Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB (2001) Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci 4:1165
Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED (2004) The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci 24:615–619
Green CB, Takahashi JS, Bass J (2008) The meter of metabolism. Cell 134:728–742
Guilding C, Piggins HD (2007) Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 25:3195–3216
Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J (2002) The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci 22:RC191
Hastings MH, Maywood ES, O’Neill JS (2008) Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol 18:R805–R815
Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424:76–81
Honma S, Ikeda M, Abe H, Tanahashi Y, Namihira M, Honma K, Nomura M (1998) Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem Biophys Res Commun 250:83–87
Hood S, Cassidy P, Cossette M-P, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S (2010) Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci 30:14046–14058
Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T (2009) Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience 158:537–544
Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F, Stehle JH (2010) Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus 20:377–388
Johnston-Wilson NL, Sims CD, Hofmann JP, Anderson L, Shore AD, Torrey EF, Yolken RH (2000) Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium. Mol Psychiatry 5:142–149
Kawaguchi Y, Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7:476–486
King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TDL, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS (1997) Positional cloning of the mouse circadian clock gene. Cell 89:641–653
Klein DC, Moore RY (1979) Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res 174:245–262
Klein DC, Bailey MJ, Carter DA, Kim JS, Shi Q, Ho AK, Chik CL, Gaildrat P, Morin F, Ganguly S, Rath MF, Møller M, Sugden D, Rangel ZG, Munson PJ, Weller JL, Coon SL (2010) Pineal function: impact of microarray analysis. Mol Cell Endocrinol 314:170–183
Lopez-Molina L, Conquet F, Dubois-Dauphin M, Schibler U (1997) The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. EMBO J 16:6762–6771
Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K-i (2000) Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci 12:4206–4214
Matsui D, Takekida S, Okamura H (2005) Molecular oscillation of Per1 and Per2 genes in the rodent brain: an in situ hybridization and molecular biological study. Kobe J Med Sci 51:85–93
Maywood ES, Chesham JE, O’Brien JA, Hastings MH (2011) A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA 108:14306–14311
Miyamoto Y, Sancar A (1998) Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci USA 95:6097–6102
Mohawk JA, Takahashi JS (2011) Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci 34:349–358
Møller M, Baeres F (2002) The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res 309:139–150
Moore RY, Eichler VB (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206
Moore RY, Lenn NJ (1972) A retinohypothalamic projection in the rat. J Comp Neurol 146:1–14
Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M, Honma K-I (1999) Daily variation and light responsiveness of mammalian clock gene, clock and BMAL1, transcripts in the pineal body and different areas of brain in rats. Neurosci Lett 267:69–72
Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, Hoeijmakers JHJ, van der Horst GTJ (1999) Photic Induction of mPer1 and mPer2 in Cry-deficient mice lacking a biological clock. Science 286:2531–2534
Onishi H, Yamaguchi S, Yagita K, Ishida Y, Dong X, Kimura H, Jing Z, Ohara H, Okamura H (2002) Rev-erbα gene expression in the mouse brain with special emphasis on its circadian profiles in the suprachiasmatic nucleus. J Neurosci Res 68:551–557
Preitner N, Damiola F, Luis Lopez M, Zakany J, Duboule D, Albrecht U, Schibler U (2002) The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260
Rath MF, Morin F, Shi Q, Klein DC, Møller M (2007) Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Exp Eye Res 85:65–73
Rath MF, Bailey MJ, Kim JS, Ho AK, Gaildrat P, Coon SL, Møller M, Klein DC (2009a) Developmental and diurnal dynamics of Pax4 expression in the mammalian pineal gland: nocturnal down-regulation is mediated by adrenergic-cyclic adenosine 3′,5′-monophosphate signaling. Endocrinology 150:803–811
Rath MF, Bailey MJ, Kim JS, Coon SL, Klein DC, Møller M (2009b) Developmental and daily expression of the Pax4 and Pax6 homeobox genes in the rat retina: localization of Pax4 in photoreceptor cells. J Neurochem 108:285–294
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941
Rovsing L, Rath MF, Lund-Andersen C, Klein DC, Møller M (2010) A neuroanatomical and physiological study of the non-image forming visual system of the cone-rod homeobox gene (Crx) knock out mouse. Brain Res 1343:54–65
Saper CB, Lu J, Chou TC, Gooley J (2005) The hypothalamic integrator for circadian rhythms. Trends Neurosci 28:152–157
Segall L, Amir S (2010) Glucocorticoid regulation of clock gene expression in the mammalian limbic forebrain. J Mol Neurosci 42:168–175
Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF Jr, Reppert SM (1997) Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19:1261–1269
Shearman LP, Zylka MJ, Reppert SM, Weaver DR (1999) Expression of basic helix-loop-helix/PAS genes in the mouse suprachiasmatic nucleus. Neuroscience 89:387–397
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM (2000) Interacting molecular loops in the mammalian circadian clock. Science 288:1013–1019
Shieh KR (2003) Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience 118:831–843
Simonneaux V, Poirel VJ, Garidou ML, Nguyen D, Diaz-Rodriguez E, Pévet P (2004) Daily rhythm and regulation of clock gene expression in the rat pineal gland. Mol Brain Res 120:164–172
Son GH, Chung S, Kim K (2011) The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol 32:451–465
Stephan FK, Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586
Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC (1997) RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90:1003–1011
Takekida S, Yan L, Maywood ES, Hastings MH, Okamura H (2000) Differential adrenergic regulation of the circadian expression of the clock genes Period1 and Period2 in the rat pineal gland. Eur J Neurosci 12:4557–4561
Takumi T, Taguchi K, Miyake S, Sakakida Y, Takashima N, Matsubara C, Maebayashi Y, Okumura K, Takekida S, Yamamoto S, Yagita K, Yan L, Young M, Okamura H (1998) A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. EMBO J 17:4753–4759
Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S (2003) Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA 100:6795–6800
Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A (1998) Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282:1490–1494
Tonsfeldt KJ, Chappell PE (2012) Clocks on top: the role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Mol Cell Endocrinol 349:3–12
Tosini G, Menaker M (1996) Circadian rhythms in cultured mammalian retina. Science 272:419–421
Tosini G, Kasamatsu M, Sakamoto K (2007) Clock gene expression in the rat retina: effects of lighting conditions and photoreceptor degeneration. Brain Res 1159:134–140
Verwey M, Amir S (2009) Food-entrainable circadian oscillators in the brain. Eur J Neurosci 30:1650–1657
Vitaterna M, King D, Chang A, Kornhauser J, Lowrey P, McDonald J, Dove W, Pinto L, Turek F, Takahashi J (1994) Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 264:719–725
Vrang N, Larsen PJ, Møller M, Mikkelsen JD (1995) Topographical organization of the rat suprachiasmatic-paraventriocular projection. J Comp Neurol 353:585–603
Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13:1190–1196
Welsh DK, Logothetis DE, Meister M, Reppert SM (1995) Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14:697–706
Wirz-Justice A (1995) Biological rhythms in mood disorders. In: Bloom F (ed) Psychopharmacology: the fourth generation of progress. Raven Press, New York, pp 999–1017
Wisor JP, Pasumarthi RK, Gerashchenko D, Thompson CL, Pathak S, Sancar A, Franken P, Lein ES, Kilduff TS (2008) Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci 28:7193–7201
Wulff K, Gatti S, Wettstein JG, Foster RG (2010) Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11:589–599
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T (2004) Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5:18
Yang S, Wang K, Valladares O, Hannenhalli S, Bucan M (2007) Genome-wide expression profiling and bioinformatics analysis of diurnally regulated genes in the mouse prefrontal cortex. Genome Biol 8:R247
Zhang EE, Kay SA (2010) Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol 11:764–776
Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400:169–173
Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC (2001) Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105:683–694
Acknowledgments
This work was supported by the Danish Medical Research Council (grants number 271-09-0206 and 271-07-0412) and the Lundbeck Foundation (grant number R34-A3364). We wish to thank Ms. Tine Thorup Mellergaard for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rath, M.F., Rohde, K., Fahrenkrug, J. et al. Circadian clock components in the rat neocortex: daily dynamics, localization and regulation. Brain Struct Funct 218, 551–562 (2013). https://doi.org/10.1007/s00429-012-0415-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0415-4