Abstract
Thymomas exhibit a unique genomic landscape, comprising the lowest on average total mutational burden among adult human cancers; a unique point mutation in the GTF2I gene in WHO type A and AB thymomas (and rarely others); almost unique KMT2A-MAML2 translocations in rare WHO type B2 and B3 thymomas; a unique YAP1-MAML2 translocation in almost all metaplastic thymomas; and unique miRNA profiles in relation to GTF2I mutational status and WHO histotypes. While most thymomas can be diagnosed solely on the basis of morphological features, mutational analyses can solve challenging differential diagnostic problems. No molecular biomarkers have been identified that predict the response of unresectable thymomas to chemotherapy or agents with known molecular targets. Despite the common and strong expression of PDL1 in thymomas, immune checkpoint inhibitors are rarely applicable due to the poor predictability of common, life-threatening autoimmune side effects that are related to the unrivaled propensity of thymomas towards autoimmunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thymomas constitute the largest subgroup (75–80%) among thymic epithelial tumors (TETs) and are the focus of this review. Thymic carcinomas (TCs) and thymic neuroendocrine tumors that constitute 10–20% and 1–2% of TETs, respectively, are reviewed elsewhere (Ströbel et al., this volume and in [1, 2]). Taking the content of immature thymocytes and the morphology of tumor cells into account, TCs are distinguished from thymomas which, in turn, are separated into WHO type A, AB, B1, B2, B3,and metaplastic thymomas as well as “micronodular thymoma with lymphoid stroma” (MNT) [3]. Apart from MNTs, TETs are malignant cancers with variable metastatic potential that increases from type A and AB, through B1, B2, and B3 thymomas to the most aggressive TCs [3]. Staging of TETs should follow the recently developed TNM system that is gradually replacing the Masaoka-Koga system [4]. Since key therapeutic guidelines still refer to the Masoaka-Koga system [5], both staging systems are still commonly used in parallel [4]. In terms of therapy, the prime aim is complete tumor resection that is usually the definite and only required intervention, while non-resectability often implies incurability [5]. However, adjuvant radiotherapy in case of uncertain resection, high tumor stage, or high-grade histology can rescue a significant number of patients [6]. In case of unresectable and recurrent thymomas, platinum-based chemotherapy is the empirical standard first-line treatment [5]. This review focuses on recent findings in the pathogenesis of thymomas and highlights gaps of knowledge that prevent efficient targeted treatment to date.
Biological features of thymomas with diagnostic and therapeutic relevance
Autoimmunity and expression of immune checkpoint molecules
Thymomas are unique tumors due to their almost consistent non-tolerogenic, intratumorous thymopoiesis that is almost never encountered in TCs and not in other carcinomas. This feature is likely the major pathomechanism that leads to the unprecedented frequency of autoimmune phenomena (about 80%) and autoimmune diseases (about 40%) in patients with thymoma but not in patients with other malignancies [7]. Among the autoimmune targets, striated muscle proteins prevail as reflected by the fact that myasthenia gravis (MG) due to autoantibodies to the Acetylcholine Receptor (AChR) and striational autoantigens (e.g., Titin, skeletal and cardiac Ryanodine Receptors (RYRs)) is the leading thymoma-associated autoimmune disease [8]. However, almost any other organ-specific (e.g., thyroid, hepatic, renal) and systemic autoimmune disease (e.g., SLE, RA) can occur either in isolation or combined with MG or other autoimmune diseases [7, 9]. The pathogenesis of most thymoma-associated autoimmune diseases is unknown. By contrast, multiomics molecular analysis revealed that thymoma-associated MG is linked to aneuploidy and over-expression of genes that encode either bona fide (e.g., AChR) or closely related (e.g., neuronal RYRs) autoimmune targets [10], while other defective tolerogenic features (e.g., the lack of AIRE expression [11,12,13,14] and defective intratumorous generation ofregulatory T cells [12, 15, 16]) might be permissive but not causative [10], although this is controversial [17]. Different molecular pathways may elicit MG in different thymoma histotypes [18].
The inclination of thymomas to autoimmune diseases has a bright diagnostic and dark therapeutic side: While preoperative detection of autoimmune features is a strong hint that a mediastinal mass is a thymoma, autoimmunity is a drawback in the era of immune interventions. Since thymomas are the cancers with the highest prevalence of abundant and strongly PDL1-expressing tumor cells [19], thymoma patients appear as ideal candidates for immunotherapies. Unfortunately, immune checkpoint inhibitors (ICIs) elicit severe autoimmune phenomena in most thymoma patients even if such phenomena are missing before therapy [2, 20]. Echoing the focus of thymoma-associated autoimmunity on striated muscle, the most life-threatening side effects of ICIs in thymoma patients are myositis, myocarditis, and MG [21,22,23]. In patients with TCs that are not “naturally” prone to autoimmunity, such side effects are less common [2, 24, 25].
Immunodeficiency in thymoma patients
Thymoma-associate acquired T cell and B cell immunodeficiencies are common and often a facet of autoimmunity. Good syndrome (in 5% of patients) is characterized by a near lack of B cells and hypogammaglobulinemia, variable CD4 T cell cytopenia, and impaired T cell activation [26]. Hypogammaglobulinemia results from autoreactive CD8+ T cells attacking B cell precursors in the bone marrow [27]. The mechanisms that elicit severe combined deficiency of CD4 and CD8 T cells [28] or the acquired hypoexpression of CD247 (encoding the CD3 zeta-chain) are unclear. CD247 hypoexpression entails susceptibility to infections [26] and, hypothetically, the increased prevalence of non-thymic cancers in thymoma patients [29]. Chronic mucocutaneous candidiasis in thymoma patients results from defective Autoimmune Regulator Gene (AIRE) expression in thymomas [11,12,13]. This elicits neutralizing autoantibodies to IL17-associated cytokines and impairs cytokine-dependent macrophage activation that, in turn, is needed to keep Candia in check [30]. Thymoma-associated immunodeficiency is a diagnostic challenge and can contribute to severe morbidity and even mortality [31,32,33,34].
The genomic landscape of thymomas
Genetic alterations in treatment naïve common thymoma types
The Cancer Genome Atlas (TCGA) consortium reported on the genetic, transcriptomic, epigenetic, miRNA and proteomic landscape of 107 thymomas (types A, AB, B1–3, MNTs) and 10 TCs from patients without prior therapy, including a high proportion of low-stage cancers [10].
In terms of somatic copy number variations, the TCGA findings were in good agreement with historic CGH studies that revealed an overall low prevalence of genomic alterations in thymomas, with particularly rare abnormalities in type A and AB compared to B2 and B3 thymomas and TCs [35,36,37]. Also, gains and losses were commonly large-scale alterations such as whole chromosome or chromosome arm losses and gains, with losses of chromosome 6 material (harboring the FOXC1 tumor suppressor gene at 6p25.3 [38]) and gains in 1q as the most common structural abnormalities across all histotypes [10, 37].
One of the most prevalent somatic mutations of thymomas is a single nucleotide hot-spot mutation (c.74146970T>A; p.L424H) in the general transcription factor IIi gene (GTF2I) [10, 39, 40]. It occurs in about 80% of type A and AB thymomas, while it is exceptionally found in type B thymomas and rare TC [10, 40]. Less common recurrent alterations concern gain-of-function mutations of HRAS (mainly in type A and AB thymomas) and NRAS (in type A and B thymomas), and loss-of-function mutations of TP53 (in type B thymomas and TCs). The enrichment of C>T mutations within CpG di-nucleotides is an age-related signature [41] that fits well with the age of thymoma patients [42]. KIT mutations and oncogenic driver mutations or translocations that are characteristic of lung and other cancers have not been observed.
Lowest total mutational burden of thymomas among adult cancers and rare MSI
On average, thymomas exhibit the lowest total mutational burden (TMB) among all adult human cancers tested in the TCGA network [41]. While a single TC among the 10 tested carcinomas showed microsatellite instability (MSI) due to a pathogenic nonsense mutation (E37*) in the MHL1 gene [41], none of the 107 tested thymomas exhibited this oncogenic feature. The latter observation may not be representative in light of our reference pathology experience (Fig. 1) and historic studies that revealed MSI in about 10% of thymomas using a PCR-based assay [19, 43].
Recurrent translocations in metaplastic thymomas
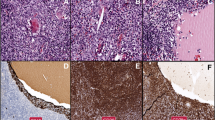
While the TCGA and other previous sequencing efforts [40, 41, 44] failed to identify recurrent translocations in type A, AB, B1–B3 thymomas and MNTs, a YAP1-MAML2 translocation (with two distinct fusion products) was recently detected by DNA RNA sequencing in all six metaplastic thymomas successfully tested so far [45]. These cases were chemotherapy naïve in accordance with their generally indolent clinical behavior [45]. Although the spindle cell component of this biphasic thymoma type (Fig. 2) vaguely resembles spindle cell areas in type A and AB thymomas [3], GTF2I mutations were consistently absent [45]. The functional effects of the YAP1-MAML2 fusion gene have not been studied but are likely oncogenic.
Metaplastic thymoma with recently described YAP1-MAML2 translocation. a Biphasic, epithelioid and spindle cell tumor; b Characteristic expression of p40 in the epithelioid but not the metaplastic/spindle cell component; c FISH analysis showing the split of the MAML2 break-apart probe (a HE stain, × 200; b immunoperoxidase, ×200; c immunofluorescence, ×400)
Recurrent translocations in rare type B2 and B3 thymomas
Recurrent KMT2A-MAML2 translocations were recently identified in 6% of clinically aggressive type B2 and B3 thymomas and a single case of combined TC (B3 thymoma with small TC component) [46]. The translocations variably involved exons 8, 9, 10, or 11 of KMT2A and exon 2 of MAML2, and are highly characteristic of type B2 and B3 thymomas, because they were previously found only in very rare leukemias, myelodysplastic syndromes, and one plasmacytoma but not in any other tumor among over 250.000 cases sequenced by Foundation Medicine, including 266 thymic carcinomas [46]. The function of the respective fusion proteins in thymomas is currently unclear, but might be oncogenic drivers, since 7 of the 11 cases did not harbor any concurrent mutations, while the four others showed only single additional mutations/variants in TP53, ARID1A, SFB1, and the TERT promoter [46]. Furthermore, KMT2A (also known as MLL) is a known oncogenic driver in translocations with other partner genes in sarcomas [47] and leukemias [46]. Since the index case of the series of Massoth et al. was a recurrent B3 thymoma biopsied after chemotherapy, and the treatment status of the other cases was not reported, it is currently unclear, whether the KMT2A-MAML2 translocation is an early or late molecular event. The latter possibility would explain why the fusion was missed in the TCGA series.
SMARCA4-deficient mediastinal/pulmonary tumors
SMARCA4-deficient cancers are a new cancer type in the upcoming WHO classification of thoracic tumors. They commonly show pleomorphic, large, and anaplastic cells, eventual deficiency of keratins, common necrosis, and defective expression of SMARCA4 (Fig. 3) or combined SMARCA4/SMARCA2 deficiency [48, 49]. So far, no bona fide thymus-restricted case has been reported, while co-invasion of thymus and lung is not uncommon. SMARCA4-deficient cancers may be confused with “thymomas with anaplasia” (see Fig. 1) that can also show defective keratin expression [50] but retain SMARCA4 expression (own observation). Since SMARCA4-deficient tumors commonly express SOX2 and SALL4 [51], mediastinal germ cell tumors also enter the differential diagnosis.
SMARCA4-deficient thoracic tumor; core needle biopsy of a mediastinal mass involving the lung (or vice versa). a Partially necrotic, undifferentiated tumor composed of large, poorly cohesive round and polygonal cells with large nuclei and prominent nucleoli; b Absence of SMARCA4 expression in the tumor cells, strong expression of SMARCA4 in endothelial cells. An identical staining pattern was seen with an antibody to SMARCA2 (not shown) (a HE stain, ×350; b immunoperoxidase)
Micro-RNAs in thymomas
Micro-RNAs (miRNAs) are non-protein-coding RNAs regulating post-transcriptional gene expression in many cancers [52], thymus development [53] and thymoma-associated autoimmunity [53,54,55]. Transcriptional overexpression of a large miRNA cluster on chromosome 19q13.42 (termed C19MC) is a common feature of type A and AB thymomas [10, 41] and associated with activation of the PI3K/AKT/mTOR pathway. Therefore, respective inhibitors might be therapeutic options in rare cases of unresectable type A and AB thymomas [41]. Another large cluster on chromosome 14 (C14MC), supposedly with tumor suppressor function, is transcriptionally silenced in many TCs [56]. In addition, various non-clustered single miRNAs are differentially expressed between thymomas and TCs [56, 57] and thought to contribute to the tumorigenesis of TCs (reviewed in [2]). So far, miRNAs do not play a role as diagnostic or therapeutic targets, and, unlike in renal cancers [58], have not been evaluated as predictive biomarkers (e.g., for sunitinib resistance).
The integrated landscape of treatment-naïve thymic epithelial tumors
Integrating TCGA data from the analysis of somatic copy number alterations, mRNA, miRNA, DNA methylation, and reverse phase protein arrays of all TETs using a “cluster-of-cluster” algorithm separated the thymomas into 3 molecular subtypes that were distinctly different from the tightly clustering TCs. As shown in Fig. 4, there was a significant overlap between the A-like and the AB-like cluster, while the members of the B-like cluster formed a continuum with minimal overlap with the AB-like cluster [10]. In agreement with previous findings [40], the GTF2I mutation was largely restricted to the A-like and AB-like clusters. In addition, the clusters segregate with the expression of key oncogenes (e.g., MYC/MAX and MYB) and suppressor genes (TP53), lymphocyte content, WHO histotype, prognosis, and MG status [10], providing strong evidence for the relevance of the WHO histological classification.
Integrated genomic landscape of thymomas and thymic carcinomas according to The Cancer Genome Atlas analysis (modified from Radovich et al. [10]). Cohorts comprise samples that are placed in the map according to similarities in their genomic profiles using all molecular platforms. The substantial overlap between the A-like and AB-like cohort indicates that quite some WHO type A and AB thymomas occur in either cohort, suggesting a molecular continuum. Little overlap between the B-like and the AB-like cohorts; of ten thymic carcinomas, one case with unique molecular features (including lack of the typical loss of 16q) was “misplaced” in the AB-like cluster. A selection of key differentially expressed molecular features is listed with each cluster. C19MC denotes a large micro-RNA cluster on chromosome 19q13.42
Genetic alterations in thymomas following chemotherapy
The TETs investigated by the TCGA consortium were chemotherapy-naïve to avoid poorly interpretably secondary genomic alteration in view of poorly standardized adjuvant therapies used to date [41]. On the other hand, targeted therapies in TETs are typically considered after the failure of various first-line treatments, making the study of post-chemotherapeutic TETs by Wang et al. even more compelling [44]. However, no recurrent genetic alterations were identified even in heavily pretreated thymomas, while TC frequently showed mutations in potential oncogenic driver genes with a role in chromatin remodeling (e.g., SMARCA4), histone modification (BAP1, SETD2, ASXL1), and DNA methylation (TET2, DNMT3a34, WT1). Mutations in these genes appear worth testing as biomarkers in TCs, as they constitute promising biomarkers in advanced renal cell carcinomas treated with sunitinib, sorafenib, and everolimus [59, 60], i.e., drugs that are used in advanced TCs (reviewed in [1]). No such perspective is currently obvious in advanced thymomas that are poor responders to sunitinib [61].
Diagnostic implications of molecular alterations in thymomas
Since most differential diagnostic problems in thymomas can be solved by morphology, the role of diagnostic molecular pathology in TETs is limited (Table 1). Exceptions may arise in small biopsies.
A GTF2I (p.L424H) mutation strongly argues for the diagnosis of type A over a focally spindly type B3 thymoma or a metaplastic thymoma [41, 45]. The distinction of atypical type A thymomas and polygonal cell-rich type A thymomas “with neuroendocrine morphology” [62] from type B3 thymomas may be other rare indications for molecular testing.
YAP1-MAML2 translocation testing is usually not necessary to diagnose metaplastic thymoma, if the biphasic nature, p40(−) spindle cell component and lack of immature T cells are taken into account [45]. Whether the derivation of some sarcomatoid carcinomas from metaplastic thymomas can be confirmed by YAP1-MAML2 testing is unknown. In small biopsies, absence of the mutation may help to confirm rare type A and AB thymomas with extensive “fibrous bands” showing an EMA(+), actin(+), and p40(−) phenotype (own observation and [63]).
The diagnostic relevance of the recently described KMT2A-MAML2 translocations in aggressive type B2 and B3 thymomas [46] needs confirmation.
Therapeutic implications of molecular alterations in thymomas and perspectives
The results of the TCGA study of thymic epithelial tumors (Table 1) confirmed previous studies that revealed absence of targetable mutations as tissue-based biomarkers in thymomas (reviewed in [1, 2]) and presence of only rare clinically meaningful mutations (e.g., of the KIT gene) in TCs [64]. In line with this, “targeted” approaches that took transcriptomic or immunohistochemical findings (e.g., overexpression of supposedly unmutated genes coding for tyrosine kinases or angiogenic factors) into account achieved rather limited success (Table 2). Accordingly, interference with other oncogenic principles (like nuclear export inhibition) is currently being investigated (reviewed in [1]). Overcoming the unacceptable frequency and severity of immune checkpoint inhibitor (ICI)-induced autoimmune side effects and simultaneously maintaining ICI therapeutic efficiency is another perspective [20]. The recently described translocations, YAP1-MAML2 and KMT2A-MAML2 in rare metaplastic and type B2 and B3 thymomas, respectively, are currently not specifically targetable either [45, 46]. However, it will be important to investigate, whether the respective fusion proteins depend in a similar way on unmutated EGFR signaling for their tumorigenic function as does the CRTC1-MAML2 fusion protein in EGFR inhibitor-sensitive mucoepidermoid carcinomas [72].
Conclusion
Uncovering many facets of the molecular landscape of thymomas has improved our understanding of pathways with relevance for oncogenesis and autoimmunity but did not reveal targets that are vulnerable to currently available therapeutic agents. It is hoped that whole genome and ex vivo single cell sequencing, the analysis of non-protein-coding RNAs, and the development of relevant model system for high throughput drug screening will overcome the current, unsatisfactory situation and advance thymoma management into the realm of truly targeted therapies.
References
Conforti F, Pala L, Giaccone G, De Pas T (2020) Thymic epithelial tumors: from biology to treatment. Cancer Treat Rev 86:102014. https://doi.org/10.1016/j.ctrv.2020.102014
Rajan A, Zhao C (2019) Deciphering the biology of thymic epithelial tumors Mediastinum (Hong Kong, China) 3. doi: https://doi.org/10.21037/med.2019.08.03
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG (2015) WHO classification of tumours of the lung, pleura, thymus and heart. IARC, Lyon
Ruffini E, Fang W, Guerrera F, Huang J, Okumura M, Kim DK, Girard N, Billè A, Boubia S, Cangir AK, Detterbeck F, Falkson C, Filosso PL, Giaccone G, Kondo K, Infante M, Lucchi M, Marino M, Marom EM, Nicholson AG, Rimner A, Rami-Porta R, Asamura H (2020) The International Association for the Study of Lung Cancer Thymic Tumors Staging Project: the impact of the eighth edition of the union for International Cancer Control and American Joint Committee on Cancer TNM stage classification of thymic tumors. J Thorac Oncol 15:436–447. https://doi.org/10.1016/j.jtho.2019.11.013
Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S (2015) Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(5):v40–v55. https://doi.org/10.1093/annonc/mdv277
Jackson MW, Palma DA, Camidge DR, Jones BL, Robin TP, Sher DJ, Koshy M, Kavanagh BD, Gaspar LE, Rusthoven CG (2017) The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol 12:734–744. https://doi.org/10.1016/j.jtho.2017.01.002
Blum TG, Misch D, Kollmeier J, Thiel S, Bauer TT (2020) Autoimmune disorders and paraneoplastic syndromes in thymoma. J Thorac dis 12:7571–7590. https://doi.org/10.21037/jtd-2019-thym-10
Gilhus NE (2016) Myasthenia gravis. N Engl J Med 375:2570–2581. https://doi.org/10.1056/NEJMra1602678
Zhao J, Bhatnagar V, Ding L, Atay SM, David EA, McFadden PM, Stamnes S, Lechtholz-Zey E, Wightman SC, Detterbeck FC, Kim AW (2020) A systematic review of paraneoplastic syndromes associated with thymoma: treatment modalities, recurrence, and outcomes in resected cases. J Thorac Cardiovasc Surg 160:306–314.e314. https://doi.org/10.1016/j.jtcvs.2019.11.052
Radovich M, Pickering CR, Felau I, Ha G, Zhang H, Jo H, Hoadley KA, Anur P, Zhang J, McLellan M, Bowlby R, Matthew T, Danilova L, Hegde AM, Kim J, MDM L, Sethi G, Lu C, Ryan M, Su X, Cherniack AD, Robertson G, Akbani R, Spellman P, Weinstein JN, Hayes DN, Raphael B, Lichtenberg T, Leraas K, Zenklusen JC, Fujimoto J, Scapulatempo-Neto C, Moreira AL, Hwang D, Huang J, Marino M, Korst R, Giaccone G, Gokmen-Polar Y, Badve S, Rajan A, Strobel P, Girard N, Tsao MS, Marx A, Tsao AS, Loehrer PJ (2018) The integrated genomic landscape of thymic epithelial tumors. Cancer Cell 33:244–258.e210. https://doi.org/10.1016/j.ccell.2018.01.003
Offerhaus GJ, Schipper ME, Lazenby AJ, Montgomery E, Morsink FH, Bende RJ, Musler AR, van Lier RA, van Noesel CJ (2007) Graft-versus-host-like disease complicating thymoma: lack of AIRE expression as a cause of non-hereditary autoimmunity? Immunol Lett 114:31–37. https://doi.org/10.1016/j.imlet.2007.08.010
Scarpino S, Di Napoli A, Stoppacciaro A, Antonelli M, Pilozzi E, Chiarle R, Palestro G, Marino M, Facciolo F, Rendina EA, Webster KE, Kinkel SA, Scott HS, Ruco L (2007) Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas. Clin Exp Immunol 149:504–512. https://doi.org/10.1111/j.1365-2249.2007.03442.x
Strobel P, Murumagi A, Klein R, Luster M, Lahti M, Krohn K, Schalke B, Nix W, Gold R, Rieckmann P, Toyka K, Burek C, Rosenwald A, Muller-Hermelink HK, Pujoll-Borrell R, Meager A, Willcox N, Peterson P, Marx A (2007) Deficiency of the autoimmune regulator AIRE in thymomas is insufficient to elicit autoimmune polyendocrinopathy syndrome type 1 (APS-1). J Pathol 211:563–571. https://doi.org/10.1002/path.2141
Suzuki E, Kobayashi Y, Yano M, Fujii Y (2008) Infrequent and low AIRE expression in thymoma: difference in AIRE expression among WHO subtypes does not correlate with association of MG. Autoimmunity 41:377–382. https://doi.org/10.1080/08916930801987573
Luther C, Poeschel S, Varga M, Melms A, Tolosa E (2005) Decreased frequency of intrathymic regulatory T cells in patients with myasthenia-associated thymoma. J Neuroimmunol 164:124–128. https://doi.org/10.1016/j.jneuroim.2005.03.011
Strobel P, Rosenwald A, Beyersdorf N, Kerkau T, Elert O, Murumagi A, Sillanpaa N, Peterson P, Hummel V, Rieckmann P, Burek C, Schalke B, Nix W, Kiefer R, Muller-Hermelink HK, Marx A (2004) Selective loss of regulatory T cells in thymomas. Ann Neurol 56:901–904. https://doi.org/10.1002/ana.20340
Liu Y, Zhang H, Zhang P, Meng F, Chen Y, Wang Y, Yao Y, Qi B (2014) Autoimmune regulator expression in thymomas with or without autoimmune disease Immunology letters. 161:50–56. https://doi.org/10.1016/j.imlet.2014.04.008
Yamada Y, Weis CA, Thelen J, Sticht C, Schalke B, Ströbel P, Marx A (2020) Thymoma associated myasthenia gravis (TAMG): differential expression of functional pathways in relation to mg status in different thymoma histotypes. Front Immunol 11:664. https://doi.org/10.3389/fimmu.2020.00664
Inaguma S, Wang Z, Lasota J, Sarlomo-Rikala M, McCue PA, Ikeda H, Miettinen M (2016) Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD-L1): analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am J Surg Pathol 40:1133–1142. https://doi.org/10.1097/pas.0000000000000653
Zhao C, Rajan A (2019) Immune checkpoint inhibitors for treatment of thymic epithelial tumors: how to maximize benefit and optimize risk? Mediastinum (Hong Kong, China) 3. doi: https://doi.org/10.21037/med.2019.08.02
Chen Q, Huang DS, Zhang LW, Li YQ, Wang HW, Liu HB (2018) Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 56:667–671. https://doi.org/10.1080/15563650.2017.1401079
Konstantina T, Konstantinos R, Anastasios K, Anastasia M, Eleni L, Ioannis S, Sofia A, Dimitris M (2019) Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung cancer 135:29–32. https://doi.org/10.1016/j.lungcan.2019.06.015
Mammen AL, Rajan A, Pak K, Lehky T, Casciola-Rosen L, Donahue RN, Lepone LM, Zekeridou A, Pittock SJ, Hassan R, Schlom J, Gulley JL (2019) Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 78:150–152. https://doi.org/10.1136/annrheumdis-2018-213777
Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ (2019) Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J clin oncol 37:2162–2170. https://doi.org/10.1200/jco.2017.77.3184
Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, Manning M, Mogg R, Blumenschein WM, Tan MT, Subramaniam DS, Liu SV, Kaplan IM, McCutcheon JN (2018) Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study The Lancet. Oncology 19:347–355. https://doi.org/10.1016/s1470-2045(18)30062-7
Christopoulos P, Dopfer EP, Malkovsky M, Esser PR, Schaefer HE, Marx A, Kock S, Rupp N, Lorenz MR, Schwarz K, Harder J, Martin SF, Werner M, Bogdan C, Schamel WW, Fisch P (2015) A novel thymoma-associated immunodeficiency with increased naive T cells and reduced CD247 expression. J immunol 194:3045–3053. https://doi.org/10.4049/jimmunol.1402805
Masci AM, Palmieri G, Vitiello L, Montella L, Perna F, Orlandi P, Abbate G, Zappacosta S, De Palma R, Racioppi L (2003) Clonal expansion of CD8+ BV8 T lymphocytes in bone marrow characterizes thymoma-associated B lymphopenia. Blood 101:3106–3108. https://doi.org/10.1182/blood-2002-08-2638
Yel L, Liao O, Lin F, Gupta S (2003) Severe T- and B-cell immune deficiency associated with malignant thymoma Annals of allergy, asthma & immunology : official publication of the American College of Allergy. Asthma, & Immunology 91:501–505. https://doi.org/10.1016/s1081-1206(10)61522-0
Welsh JS, Howard SP (2015) Comment on “A novel thymoma-associated immunodeficiency with increased naive t cells and reduced CD247 expression”. J Immunol 195(1950):3505–353505. https://doi.org/10.4049/jimmunol.1501667
Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Ströbel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A (2010) Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207:299–308. https://doi.org/10.1084/jem.20091669
Holbro A, Jauch A, Lardinois D, Tzankov A, Dirnhofer S, Hess C (2012) High prevalence of infections and autoimmunity in patients with thymoma. Hum Immunol 73:287–290. https://doi.org/10.1016/j.humimm.2011.12.022
Okusu T, Sato T, Ogata Y, Nagata S, Kozumi K, Kim SH, Yamamoto S, Yamayoshi S (2016) Good’s syndrome accompanied by agranulocytosis following a rapid clinical course. Int med 55:537–540. https://doi.org/10.2169/internalmedicine.55.5542
Santos E, Silva AM, Stroebel P, Marinho A, Willcox N, Goncalves G, Lopes C, Marx A, Leite MI (2019) Signs heralding appearance of thymomas after extended thymectomy for myasthenia gravis Neurology. Clinical practice 9:48–52. https://doi.org/10.1212/cpj.0000000000000551
Zaman M, Huissoon A, Buckland M, Patel S, Alachkar H, Edgar JD, Thomas M, Arumugakani G, Baxendale H, Burns S, Williams AP, Jolles S, Herriot R, Sargur RB, Arkwright PD (2019) Clinical and laboratory features of seventy-eight UK patients with Good’s syndrome (thymoma and hypogammaglobulinaemia). Clin Exp Immunol 195:132–138. https://doi.org/10.1111/cei.13216
Inoue M, Starostik P, Zettl A, Ströbel P, Schwarz S, Scaravilli F, Henry K, Willcox N, Müller-Hermelink HK, Marx A (2003) Correlating genetic aberrations with World Health Organization-defined histology and stage across the spectrum of thymomas. Cancer Res 63:3708–3715
Penzel R, Hoegel J, Schmitz W, Blaeker H, Morresi-Hauf A, Aulmann S, Hecker E, Mechtersheimer G, Otto HF, Rieker RJ (2003) Clusters of chromosomal imbalances in thymic epithelial tumours are associated with the WHO classification and the staging system according to Masaoka. Int J Cancer 105:494–498. https://doi.org/10.1002/ijc.11101
Zettl A, Ströbel P, Wagner K, Katzenberger T, Ott G, Rosenwald A, Peters K, Krein A, Semik M, Müller-Hermelink HK, Marx A (2000) Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol 157:257–266. https://doi.org/10.1016/s0002-9440(10)64536-1
Petrini I, Wang Y, Zucali PA, Lee HS, Pham T, Voeller D, Meltzer PS, Giaccone G (2013) Copy number aberrations of genes regulating normal thymus development in thymic epithelial tumors. Clin Cancer Res 19:1960–1971. https://doi.org/10.1158/1078-0432.ccr-12-3260
Feng Y, Lei Y, Wu X, Huang Y, Rao H, Zhang Y, Wang F (2017) GTF2I mutation frequently occurs in more indolent thymic epithelial tumors and predicts better prognosis. Lung cancer 110:48–52. https://doi.org/10.1016/j.lungcan.2017.05.020
Petrini I, Meltzer PS, Kim IK, Lucchi M, Park KS, Fontanini G, Gao J, Zucali PA, Calabrese F, Favaretto A, Rea F, Rodriguez-Canales J, Walker RL, Pineda M, Zhu YJ, Lau C, Killian KJ, Bilke S, Voeller D, Dakshanamurthy S, Wang Y, Giaccone G (2014) A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 46:844–849. https://doi.org/10.1038/ng.3016
Radovich M, Solzak JP, Hancock BA, Conces ML, Atale R, Porter RF, Zhu J, Glasscock J, Kesler KA, Badve SS, Schneider BP, Loehrer PJ (2016) A large microRNA cluster on chromosome 19 is a transcriptional hallmark of WHO type A and AB thymomas. Br J Cancer 114:477–484. https://doi.org/10.1038/bjc.2015.425
Roden AC, Fang W, Shen Y, Carter BW, White DB, Jenkins SM, Spears GM, Molina JR, Klang E, Segni MD, Ackman JB, Sanchez EZ, Girard N, Shumeri E, Revel MP, Chassagnon G, Rubinowitz A, Dicks D, Detterbeck F, Ko JP, Falkson CB, Sigurdson S, Segreto S, Del Vecchio S, Palmieri G, Ottaviano M, Marino M, Korst R, Marom EM (2020) Distribution of mediastinal lesions across multi-institutional, international, radiology databases Journal of thoracic oncology : official publication of the International Association for the Study of. Lung Cancer 15:568–579. https://doi.org/10.1016/j.jtho.2019.12.108
Inoue M, Marx A, Zettl A, Ströbel P, Müller-Hermelink HK, Starostik P (2002) Chromosome 6 suffers frequent and multiple aberrations in thymoma. Am J Pathol 161:1507–1513. https://doi.org/10.1016/s0002-9440(10)64426-4
Wang Y, Thomas A, Lau C, Rajan A, Zhu Y, Killian JK, Petrini I, Pham T, Morrow B, Zhong X, Meltzer PS, Giaccone G (2014) Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Report 4:7336. https://doi.org/10.1038/srep07336
Vivero M, Davineni P, Nardi V, Chan JKC, Sholl LM (2020) Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions Modern pathology : an official journal of the United States and Canadian Academy of Pathology. Inc 33:560–565. https://doi.org/10.1038/s41379-019-0382-x
Massoth LR, Hung YP, Dias-Santagata D, Onozato M, Shah N, Severson E, Duncan D, Gillespie BJ, Williams NF, Ross JS, Vergilio JA, Harkins SK, Glomski K, Nardi V, Zukerberg LR, Hasserjian RP, Louissaint A Jr, Williams EA (2020) Pan-cancer landscape analysis reveals recurrent KMT2A-MAML2 gene fusion in aggressive histologic subtypes of thymoma. JCO precision oncology 4:109–115. https://doi.org/10.1200/po.19.00288
Massoth LR, Hung YP, Nardi V, Nielsen GP, Hasserjian RP, Louissaint A Jr, Fisch AS, Deshpande V, Zukerberg LR, Lennerz JK, Selig M, Glomski K, Patel PJ, Williams KJ, Sokol ES, Alexander BM, Vergilio JA, Ross JS, Pavlick DC, Chebib I, Williams EA (2020) Pan-sarcoma genomic analysis of KMT2A rearrangements reveals distinct subtypes defined by YAP1-KMT2A-YAP1 and VIM-KMT2A fusions Modern pathology : an official journal of the United States and Canadian Academy of Pathology. Inc 33:2307–2317. https://doi.org/10.1038/s41379-020-0582-4
Le Loarer F, Watson S, Pierron G, de Montpreville VT, Ballet S, Firmin N, Auguste A, Pissaloux D, Boyault S, Paindavoine S, Dechelotte PJ, Besse B, Vignaud JM, Brevet M, Fadel E, Richer W, Treilleux I, Masliah-Planchon J, Devouassoux-Shisheboran M, Zalcman G, Allory Y, Bourdeaut F, Thivolet-Bejui F, Ranchere-Vince D, Girard N, Lantuejoul S, Galateau-Sallé F, Coindre JM, Leary A, Delattre O, Blay JY, Tirode F (2015) SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 47:1200–1205. https://doi.org/10.1038/ng.3399
Rekhtman N, Montecalvo J, Chang JC, Alex D, Ptashkin RN, Ai N, Sauter JL, Kezlarian B, Jungbluth A, Desmeules P, Beras A, Bishop JA, Plodkowski AJ, Gounder MM, Schoenfeld AJ, Namakydoust A, Li BT, Rudin CM, Riely GJ, Jones DR, Ladanyi M, Travis WD (2020) SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol 15:231–247. https://doi.org/10.1016/j.jtho.2019.10.023
Adam P, Hakroush S, Hofmann I, Reidenbach S, Marx A, Strobel P (2014) Thymoma with loss of keratin expression (and giant cells): a potential diagnostic pitfall. Virchows Arch 465:313–320. https://doi.org/10.1007/s00428-014-1606-6
Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, Yvorel V, Pissaloux D, Thomas de Montpreville V, Masliah-Planchon J, Lantuejoul S, Brevet M, Blay JY, Coindre JM, Tirode F, Le Loarer F (2019) SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol 43:455–465. https://doi.org/10.1097/pas.0000000000001188
Farooqi AA, Fuentes-Mattei E, Fayyaz S, Raj P, Goblirsch M, Poltronieri P, Calin GA (2019) Interplay between epigenetic abnormalities and deregulated expression of microRNAs in cancer. Semin Cancer Biol 58:47–55. https://doi.org/10.1016/j.semcancer.2019.02.003
Cron MA, Guillochon É, Kusner L, Le Panse R (2020) Role of miRNAs in normal and myasthenia gravis thymus Frontiers in immunology. 11:1074. https://doi.org/10.3389/fimmu.2020.01074
Li J, Qiu D, Chen Z, Du W, Liu J, Mo X (2016) Altered expression of miR-125a-5p in thymoma-associated myasthenia gravis and its down-regulation of foxp3 expression in Jurkat cells. Immunol Lett 172:47–55. https://doi.org/10.1016/j.imlet.2016.02.005
Wang Z, Chen Y, Xu S, Yang Y, Wei D, Wang W, Huang X (2015) Aberrant decrease of microRNA19b regulates TSLP expression and contributes to Th17 cells development in myasthenia gravis related thymomas. J Neuroimmunol 288:34–39. https://doi.org/10.1016/j.jneuroim.2015.08.013
Enkner F, Pichlhöfer B, Zaharie AT, Krunic M, Holper TM, Janik S, Moser B, Schlangen K, Neudert B, Walter K, Migschitz B, Müllauer L (2017) Molecular profiling of thymoma and thymic carcinoma: genetic differences and potential novel therapeutic targets Pathology oncology research. POR 23:551–564. https://doi.org/10.1007/s12253-016-0144-8
Ganci F, Vico C, Korita E, Sacconi A, Gallo E, Mori F, Cambria A, Russo E, Anile M, Vitolo D, Pescarmona E, Blandino R, Facciolo F, Venuta F, Blandino G, Marino M, Fazi F (2014) MicroRNA expression profiling of thymic epithelial tumors. Lung cancer 85:197–204. https://doi.org/10.1016/j.lungcan.2014.04.008
Sekino Y, Sakamoto N, Sentani K, Oue N, Teishima J, Matsubara A, Yasui W (2019) miR-130b promotes sunitinib resistance through regulation of PTEN in renal cell carcinoma. Oncology 97:164–172. https://doi.org/10.1159/000500605
Sekino Y, Hagura T, Han X, Babasaki T, Goto K, Inoue S, Hayashi T, Teishima J, Shigeta M, Taniyama D, Kuraoka K, Sentani K, Yasui W, Matsubara A (2020) PTEN Is involved in sunitinib and sorafenib resistance in renal cell carcinoma. Anticancer Res 40:1943–1951. https://doi.org/10.21873/anticanres.14149
Voss MH, Reising A, Cheng Y, Patel P, Marker M, Kuo F, Chan TA, Choueiri TK, Hsieh JJ, Hakimi AA, Motzer RJ (2018) Genomically annotated risk model for advanced renal-cell carcinoma: a retrospective cohort study The Lancet. Oncology 19:1688–1698. https://doi.org/10.1016/s1470-2045(18)30648-x
Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, Lee MJ, Lee S, Ling A, Spittler AJ, Carter CA, Guha U, Wang Y, Szabo E, Meltzer P, Steinberg SM, Trepel JB, Loehrer PJ, Giaccone G (2015) Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial The Lancet. Oncology 16:177–186. https://doi.org/10.1016/s1470-2045(14)71181-7
Weissferdt A, Moran CA (2014) Spindle cell thymomas with neuroendocrine morphology: a clinicopathological and immunohistochemical study of 18 cases. Histopathology 65:111–118. https://doi.org/10.1111/his.12376
Weissferdt A, Hernandez JC, Kalhor N, Moran CA (2011) Spindle cell thymomas: an immunohistochemical study of 30 cases Applied immunohistochemistry & molecular morphology. AIMM 19:329–335. https://doi.org/10.1097/PAI.0b013e318203baa1
Hirai F, Edagawa M, Shimamatsu S, Toyozawa R, Toyokawa G, Nosaki K, Yamaguchi M, Seto T, Twakenoyama M, Ichinose Y (2016) c-kit mutation-positive advanced thymic carcinoma successfully treated as a mediastinal gastrointestinal stromal tumor. Mol Clin Oncol 4:527–529. https://doi.org/10.3892/mco.2016.752
Besse B, Garassino MC, Rajan A, Novello S, Mazieres J, Weiss GJ, Kocs DM, Barnett JM, Davite C, Crivori P, Giaccone G (2018) Efficacy of milciclib (PHA-848125AC), a pan-cyclin d-dependent kinase inhibitor, in two phase II studies with thymic carcinoma (TC) and B3 thymoma (B3T) patients. J Clin Oncol 36:8519–8519. https://doi.org/10.1200/JCO.2018.36.15_suppl.8519
Giaccone G, Rajan A, Berman A, Kelly RJ, Szabo E, Lopez-Chavez A, Trepel J, Lee MJ, Cao L, Espinoza-Delgado I, Spittler J, Loehrer PJ Sr (2011) Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol 29:2052–2059. https://doi.org/10.1200/jco.2010.32.4467
Gubens MA, Burns M, Perkins SM, Pedro-Salcedo MS, Althouse SK, Loehrer PJ, Wakelee HA (2015) A phase II study of saracatinib (AZD0530), a Src inhibitor, administered orally daily to patients with advanced thymic malignancies. Lung Cancer 89:57–60. https://doi.org/10.1016/j.lungcan.2015.04.008
Kurup A, Burns M, Dropcho S, Pao W, Loehrer PJ (2005) Phase II study of gefitinib treatment in advanced thymic malignancies. J Clin Oncol 23:7068–7068. https://doi.org/10.1200/jco.2005.23.16_suppl.7068
Palmieri G, Marino M, Buonerba C, Federico P, Conti S, Milella M, Petillo L, Evoli A, Lalle M, Ceribelli A, Merola G, Matano E, Sioletic S, De Placido S, Di Lorenzo G, Damiano V (2012) Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother Pharmacol 69:309–315. https://doi.org/10.1007/s00280-011-1690-0
Zaid MIA, Radovich M, Althouse SK, Liu H, Spittler AJ, Solzak JP, Hancock BA, Loehrer PJ (2018) A phase II study of BKM120 (buparlisib) in relapsed or refractory thymomas. J Clin Oncol 36:e20580–e20580. https://doi.org/10.1200/JCO.2018.36.15_suppl.e20580
Zucali PA, De Pas T, Palmieri G, Favaretto A, Chella A, Tiseo M, Caruso M, Simonelli M, Perrino M, De Vincenzo F, Toffalorio F, Damiano V, Pasello G, Garbella E, Ali M, Conforti F, Ottaviano M, Cioffi A, De Placido S, Giordano L, Bertossi M, Destro A, Di Tommaso L, Santoro A (2018) phase ii study of everolimus in patients with thymoma and thymic carcinoma previously treated with cisplatin-based chemotherapy. Journal of clinical oncology. J Clin Oncol 36:342–349. https://doi.org/10.1200/jco.2017.74.4078
Wu Y, He Z, Li S, Tang H, Wang L, Yang S, Dong B, Qin J, Sun Y, Yu H, Zhang Y, Zhang Y, Guo Y, Wang Q (2019) Gefitinib represses JAK-STAT signaling activated by CRTC1-MAML2 fusion in mucoepidermoid carcinoma cells. Curr Cancer Drug Targets 19:796–806. https://doi.org/10.2174/1568009619666190103122735
Author information
Authors and Affiliations
Contributions
Prof. A. Marx and Dr. Y. Yamada designed and wrote the manuscript; Dr. D. Belharazem was in charge of the screening of literature on molecular thymoma features; Dr. D-H. Lee and Prof. Schalke, Neurology, were in charge of neurological/myasthenia gravis-related thymoma issues; Z. V. Popovic and C-A Weis were in charge of image processing and design; Prof. Reißfelder and Prof. Schölch, Surgery, provided key tumor material and information on thymoma patients; Prof. P. Ströbel covered thymoma-related autoimmunity and differential diagnostic issues. All authors critically reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Morphological evaluation of archival material from the Institute of Pathology, University Medical Centre Mannheim, does not require patient consent as approved by the Medical Ethics Committee II, Medical Faculty Mannheim, Heidelberg University (ethics approval # 2017-806R-MA).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marx, A., Belharazem, D., Lee, DH. et al. Molecular pathology of thymomas: implications for diagnosis and therapy. Virchows Arch 478, 101–110 (2021). https://doi.org/10.1007/s00428-021-03068-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03068-8