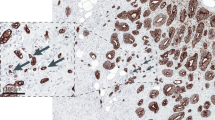
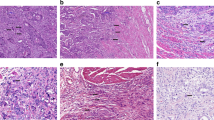
Abstract
Budding is a process during which individual or small clusters of up to five tumour cells detach from the main tumour mass and invade into the surrounding stroma. In colorectal cancer, this feature is observed in 20–40 % of cases and is associated with lymphovascular invasion, lymph node and distant metastases, and poor prognosis. A variety of scoring systems for budding have been proposed but so far a gold standard is lacking, hampering implementation of a budding score in guidelines for pathological examination of colorectal cancer. Furthermore, little is known about the mechanisms which cause tumour cells to detach from the main tumour mass and obtain increased invasive potential. In this review, we present an overview of tumour budding including its definition, scoring systems, prognostic relevance and biological mechanisms involved.


Similar content being viewed by others
References
Imai T (1954) The growth of human carcinoma. A morphological analysis. Fukuoka Igaku Zasshi 45:13–43
Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M (1989) An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 63:539–543
Hase K, Shatney C, Johnson D, Trollope M, Vierra M (1993) Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 36:627–635
Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC (2002) Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40:127–132
Prall F, Nizze H, Barten M (2005) Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology 47:17–24
Caie PD, Turnbull AK, Farrington SM, Oniscu A, Harrison DJ (2014) Quantification of tumour budding, lymphatic vessel density and invasion through image analysis in colorectal cancer. J Transl Med 12:156
Puppa G, Senore C, Sheahan K, Vieth M, Lugli A, Zlobec I, Pecori S, Wang LM, Langner C, Mitomi H, et al. (2012) Diagnostic reproducibility of tumour budding in colorectal cancer: a multicentre, multinational study using virtual microscopy. Histopathology 61:562–575
Karamitopoulou E, Zlobec I, Kölzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, Lugli A (2013) Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 26:295–301
Lugli A, Karamitopoulou E, Zlobec I (2012) Tumour budding: a promising parameter in colorectal cancer. Br J Cancer 106:1713–1717
Koelzer VH, Zlobec I, Berger MD, Cathomas G, Dawson H, Dirschmid K, Hädrich M, Inderbitzin D, Offner F, Puppa G, et al. (2015) Tumor budding in colorectal cancer revisited: results of a multicenter interobserver study. Virchows Arch 466:485–493
Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY (2009) Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum 52:438–445
Yamauchi H, Togashi K, Kawamura YJ, Horie H, Sasaki J, Tsujinaka S, Yasuda Y, Konishi F (2008) Pathological predictors for lymph node metastasis in T1 colorectal cancer. Surg Today 38:905–910
Losi L, Ponti G, Gregorio CD, Marino M, Rossi G, Pedroni M, Benatti P, Roncucci L, de Leon MP (2006) Prognostic significance of histological features and biological parameters in stage I (pT1 and pT2) colorectal adenocarcinoma. Pathol Res Pract 202:663–670
Wang HS, Liang WY, Lin TC, Chen WS, Jiang JK, Yang SH, Chang SC, Lin JK (2005) Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum 48:1182–1192
Okuyama T, Nakamura T, Yamaguchi M (2003) Budding is useful to select high-risk patients in stage II well-differentiated or moderately differentiated colon adenocarcinoma. Dis Colon Rectum 46:1400–1406
Nakamura T, Mitomi H, Kanazawa H, Ohkura Y, Watanabe M (2008) Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis Colon Rectum 51:568–572
Tanaka M, Hashiguchi Y, Ueno H, Hase K, Mochizuki H (2003) Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum 46:1054–1059
Betge J, Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Vieth M, Langner C (2012) Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann Surg Oncol 19:3706–3712
Sy J, Fung CL, Dent OF, Chapuis PH, Bokey L, Chan C (2010) Tumor budding and survival after potentially curative resection of node-positive colon cancer. Dis Colon Rectum 53:301–307
Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, Lynch CF, French AJ, Slager SL, Raissian Y, et al. (2015) Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol 39:1340–1346
Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, Lugli A (2013) Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol 44:697–705
Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C (2005) Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum 48:1597–1602
Ha SS, Choi HJ, Park KJ, Kim JM, Kim SH, Roh YH, Kwon HC, Roh MS (2005) Intensity of tumor budding as an index for the malignant potential in invasive rectal carcinoma. Cancer Res Treat 37:177–182
Langner C, Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Schlemmer A, Vieth M, Rehak P (2012) Mucinous differentiation in colorectal cancer—indicator of poor prognosis? Histopathology 60:1060–1072
Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, Nakagawa T, Uchimoto K, Nakamura S, Nonomura A, Nakajima Y (2008) Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res 28:1831–1836
Wöhlke M, Schiffmann L, Prall F (2011) Aggressive colorectal carcinoma phenotypes of invasion can be assessed reproducibly and effectively predict poor survival: interobserver study and multivariate survival analysis of a prospectively collected series of 299 patients after potentially curative resections with long-term follow-up. Histopathology 59:857–866
Zlobec I, Hädrich M, Dawson H, Koelzer VH, Borner M, Mallaev M, Schnüriger B, Inderbitzin D, Lugli A (2014) Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br J Cancer 110:1008–1013
Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z (2015) The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol 141:189–201
Peng Z, Wang CX, Fang EH, Wang GB, Tong Q (2014) Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol 20:5403–5410
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196
Xu J, Lamouille S, Derynck R (2009) TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19:156–172
Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR (2007) Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol 212:260–268
Talbot LJ, Bhattacharya SD, Kuo PC (2012) Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol 3:117–136
Zlobec I, Lugli A (2010) Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 1:651–661
García-Solano J, Conesa-Zamora P, Trujillo-Santos J, Torres-Moreno D, Mäkinen MJ, Pérez-Guillermo M (2012) Immunohistochemical expression profile of β-catenin, E-cadherin, P-cadherin, laminin-5γ2 chain, and SMAD4 in colorectal serrated adenocarcinoma. Hum Pathol 43:1094–1102
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T (2001) Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 98:10356–10361
Roca F, Mauro LV, Morandi A, Bonadeo F, Vaccaro C, Quintana GO, Specterman S, de Kier Joffé EB, Pallotta MG, Puricelli LI, Lastiri J (2006) Prognostic value of E-cadherin, beta-catenin, MMPs (7 and 9), and TIMPs (1 and 2) in patients with colorectal carcinoma. J Surg Oncol 93:151–160
Pyke C, Salo S, Ralfkiaer E, Rømer J, Danø K, Tryggvason K (1995) Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res 55:4132–4139
Guzińska-Ustymowicz K (2006) MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC). Anticancer Res 26:1589–1594
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G (2005) The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res 65:6237–6244
Ikenouchi J, Matsuda M, Furuse M, Tsukita S (2003) Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116:1959–1967
Yusra SS, Yokozaki H (2012) Biological significance of tumor budding at the invasive front of human colorectal carcinoma cells. Int J Oncol 41:201–210
Kim YH, Kim G, Kwon CI, Kim JW, Park PW, Hahm KB (2014) TWIST1 and SNAI1 as markers of poor prognosis in human colorectal cancer are associated with the expression of ALDH1 and TGF-β1. Oncol Rep 31:1380–1388
Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G (2005) SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res 33:6566–6578
Tania M, Khan MA, Fu J (2014) Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol 35:7335–7342
Ono H, Imoto I, Kozaki K, Tsuda H, Matsui T, Kurasawa Y, Muramatsu T, Sugihara K, Inazawa J (2012) SIX1 promotes epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation. Oncogene 31:4923–4934
Zhang GJ, Zhou T, Tian HP, Liu ZL, Xia SS (2013) High expression of ZEB1 correlates with liver metastasis and poor prognosis in colorectal cancer. Oncol Lett 5:564–568
Kahlert C, Lahes S, Radhakrishnan P, Dutta S, Mogler C, Herpel E, Brand K, Steinert G, Schneider M, Mollenhauer M, et al. (2011) Overexpression of ZEB2 at the invasion front of colorectal cancer is an independent prognostic marker and regulates tumor invasion in vitro. Clin Cancer Res 17:7654–7663
Galván JA, Astudillo A, Vallina A, Crespo G, Folgueras MV, González MV (2014) Prognostic and diagnostic value of epithelial to mesenchymal transition markers in pulmonary neuroendocrine tumors. BMC Cancer 14:855
Jass JR, Young J, Leggett BA (2002) Evolution of colorectal cancer: change of pace and change of direction. J Gastroenterol Hepatol 17:17–26
Dawson H, Lugli A (2015) Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med (Lausanne) 2:11
Sánchez-Tilló E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A (2011) β-Catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A 108:19204–19209
Brabletz T, Jung A, Hermann K, Günther K, Hohenberger W, Kirchner T (1998) Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract 194:701–704
Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, Niedobitek G, Brabletz T, Kirchner T (2001) The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol 159:1613–1617
Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, Ben-Ze’ev A (2005) L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 168:633–642
El-Gendi S, Al-Gendi A (2011) Assessment of tumor budding in colorectal carcinoma: correlation with β-catenin nuclear expression. J Egypt Natl Canc Inst 23:1–9
Hörkkö TT, Klintrup K, Mäkinen JM, Näpänkangas JB, Tuominen HJ, Mäkelä J, Karttunen TJ, Mäkinen MJ (2006) Budding invasive margin and prognosis in colorectal cancer—no direct association with beta-catenin expression. Eur J Cancer 42:964–971
Gao ZH, Lu C, Wang MX, Han Y, Guo LJ (2014) Differential β-catenin expression levels are associated with morphological features and prognosis of colorectal cancer. Oncol Lett 8:2069–2076
Chen Z, He X, Jia M, Liu Y, Qu D, Wu D, Wu P, Ni C, Zhang Z, Ye J, et al. (2013) β-catenin overexpression in the nucleus predicts progress disease and unfavourable survival in colorectal cancer: a meta-analysis. PLoS One 8:e63854
Brabletz T, Spaderna S, Kolb J, Hlubek F, Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C, et al. (2004) Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I: an active role for the tumor environment in malignant tumor progression. Cancer Res 64:6973–6977
Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B (1998) Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol 185:44–52
Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T (2004) Beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer 108:321–326
Hlubek F, Jung A, Kotzor N, Kirchner T, Brabletz T (2001) Expression of the invasion factor laminin gamma2 in colorectal carcinomas is regulated by beta-catenin. Cancer Res 61:8089–8093
Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y (2002) Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene 21:5861–5867
Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V (2000) Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol 148:615–624
Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID (2007) Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol 20:221–232
Karamitopoulou E (2013) Role of epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma: is tumor budding the missing link? Front Oncol 3:221
Dawson H, Koelzer VH, Karamitopoulou E, Economou M, Hammer C, Muller DE, Lugli A, Zlobec I (2014) The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology 64:577–584
Koelzer VH, Karamitopoulou E, Dawson H, Kondi-Pafiti A, Zlobec I, Lugli A (2013) Geographic analysis of RKIP expression and its clinical relevance in colorectal cancer. Br J Cancer 108:2088–2096
Karamitopoulou E, Zlobec I, Gloor B, Kondi-Pafiti A, Lugli A, Perren A (2013) Loss of Raf-1 kinase inhibitor protein (RKIP) is strongly associated with high-grade tumor budding and correlates with an aggressive phenotype in pancreatic ductal adenocarcinoma (PDAC). J Transl Med 11:311
Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM (2011) mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res 71:3246–3256
Hostettler L, Zlobec I, Terracciano L, Lugli A (2010) ABCG5-positivity in tumor buds is an indicator of poor prognosis in node-negative colorectal cancer patients. World J Gastroenterol 16:732–739
Zlobec I, Minoo P, Terracciano L, Baker K, Lugli A (2011) Characterization of the immunological microenvironment of tumour buds and its impact on prognosis in mismatch repair-proficient and -deficient colorectal cancers. Histopathology 59:482–495
Kevans D, Wang LM, Sheahan K, Hyland J, O’Donoghue D, Mulcahy H, O’Sullivan J (2011) Epithelial-mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol 19:751–760
Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M, Terracciano L, Zlobec I (2009) CD8+ lymphocytes/tumour-budding index: an independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br J Cancer 101:1382–1392
Steinestel K, Lennerz JK, Eder S, Kraft K, Arndt A (2014) Invasion pattern and histologic features of tumor aggressiveness correlate with MMR protein expression, but are independent of activating KRAS and BRAF mutations in CRC. Virchows Arch 465:155–163
Prall F, Ostwald C (2007) High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum Pathol 38:1696–1702
De Smedt L, Lemahieu J, Palmans S, Govaere O, Tousseyn T, Van Cutsem E, Prenen H, Tejpar S, Spaepen M, Matthijs G, et al. (2015) Microsatellite instable vs stable colon carcinomas: analysis of tumour heterogeneity, inflammation and angiogenesis. Br J Cancer 113:500–509
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
De Smedt, L., Palmans, S. & Sagaert, X. Tumour budding in colorectal cancer: what do we know and what can we do?. Virchows Arch 468, 397–408 (2016). https://doi.org/10.1007/s00428-015-1886-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1886-5