Abstract
Malignant mesothelioma, an aggressive and often lethal tumor commonly associated with asbestos exposure, has been morphologically classified into epithelial, biphasic, and sarcomatoid subtypes. Histological distinction between biphasic or sarcomatoid mesothelioma and synovial sarcoma may be problematic in certain circumstances of intrathoracic location because of their similar clinicopathologic features, including not only their morphology but also occasional positive immunoreaction of mesothelioma markers. TLE1, which plays an important role in Wnt pathway, has been shown to be a specific marker for synovial sarcoma and diagnostically is useful; however, TLE1 expression in malignant mesotheliomas has not been fully evaluated. We immunohistochemically examined the expression of TLE1, factors related to the Wnt pathway including β-catenin and cyclin D1, and mesothelioma markers including calretinin, HBME-1, cytokeratin 5/6, and thrombomodulin in 29 malignant mesotheliomas. TLE1 was variably expressed in 28 malignant mesotheliomas regardless of histomorphological subtype with >25% of positive cells in 20 cases (69.0%). There was no evidence of association of TLE1 expression with immunoreactivity to other markers. Our study showed no or limited value of the immunohistochemical TLE1 expression in distinguishing malignant mesothelioma and synovial sarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant mesothelioma is an aggressive, often lethal tumor commonly associated with asbestos exposure [1]. The 2004 World Health Organization classification broadly classifies malignant mesothelioma into three subtypes: epithelial, biphasic, and sarcomatoid mesothelioma [1]. In contrast to epithelioid mesothelioma, which may have histological features reminiscent of carcinoma, biphasic, and sarcomatoid mesotheliomas should be histologically differentiated from a variety of tumors, including sarcomatoid carcinoma or mesenchymal tumors. Synovial sarcoma is one of the most problematic differential diagnoses of biphasic or sarcomatoid malignant mesothelioma in certain circumstances of pleural and pulmonary locations [2, 3] because of their overlapping monophasic fibrous or biphasic epithelioid and sarcomatoid morphology and occasional positive immunoreaction of some mesothelioma markers [4]. Demonstration of the reciprocal translocation t(X;18)(p11;q11) or the resulting SS18–SSX gene fusion by cytogenetics, reverse transcription-polymerase chain reaction or fluorescence in situ hybridization has proven specific for synovial sarcoma and diagnostically useful [1]; however, assessments for these genetic aberrations require an apparatus or certain techniques for genetic or molecular assays, which are not always available in pathological departments or laboratories. It may be practically valuable to make a correct diagnosis without such an ancillary molecular technique. TLE1, which plays an important role in the Wnt pathway [5], has been shown to be a specific and diagnostically useful immunohistochemical marker for synovial sarcoma [6]. Subsequently, TLE1 expression has been demonstrated even in a subset of soft tissue tumors other than synovial sarcoma [7–9] and might play a limited role in diagnosing synovial sarcoma; however, there have been few reports of the status of TLE1 in malignant mesotheliomas [8].
In the present study, we immunohistochemically evaluated the expression of TLE1 in a series of malignant mesothelioma to assess its value in differentiating malignant mesothelioma from synovial sarcoma. Expressions of mesothelioma markers and factors associated with the Wnt pathway were also examined in both types of tumors.
Materials and methods
Twenty-nine lesions of malignant mesothelioma were retrieved from the files of the Department of Pathology, University of Occupational and Environmental Health Hospital, and of the Department of Pathology and Oncology, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan. They were clinicopathologically and immunohistochemically re-evaluated, and subclassified into the three histological variants: epithelioid, sarcomatoid, and biphasic. Biphasic mesothelioma was defined as tumor with more than 10% of each epithelioid or sarcomatoid component [1].
For the immunohistochemical analysis, Histofine Simple Stain MAX PO (Nichirei, Tokyo, Japan) employing the universal immunoenzyme amino acid polymer method was used. The TLE1 antibody (sc-9121, rabbit polyclonal, 1:20; Santa Cruz, CA, USA) used and its dilution were described in previous studies [6, 8, 9]. We preliminarily tested several standard pretreatment methods, including those used in the previous studies. As a result, heating in 10 mmol/L citrate buffer at pH 6.0 by a pressure cooker for 10 min was adopted because of its most constant and intense staining results. Other antibodies used were as follows: β-catenin (14/beta-catenin, monoclonal, 1:100; BD Transduction Laboratories, San Diego, CA, USA), cyclin D1 (SP4, rabbit monoclonal, 1:50; Nichirei), calretinin (DC8, rabbit polyclonal, 1:1; Zymed, south San Francisco, CA, USA), cytokeratins 5/6 (D5/16 B4, monoclonal, 1:50; Dako, Glostrup, Denmark), thrombomodulin (TM-1009, monoclonal, 1:25; Dako), and HBME-1 (monoclonal, 1:100; Dako). For antigen retrieval, heating in 10 mmol/L citrate buffer (pH 6.0) by a pressure cooker was performed for 10 min before the immunoreaction for β-catenin and cyclin D1 or in 1 mM ethylenediamine tetraacetic acid (EDTA) solution at pH 8.0 for calretinin and cytokeratin 5/6. A protease treatment was performed for thrombomodulin. After the incubation with the primary antibodies at room temperature, the reaction products were visualized by soaking sections in 0.2 mg/mL diaminobenzidine in 0.05 mol/L Tris–HCl buffer, pH 7.6, containing 0.003% hydrogen peroxide. For an evaluation of the TLE1 expression in malignant mesotheliomas, we examined 114 slides derived from 20 biopsy, 20 surgical, and two autopsy specimens (one to seven slides per specimen), including those of recurrent or metastatic tumors because TLE1 expression was reported to be potentially heterogeneous within the same tumor [9]. We evaluated the immunoreactivity of the markers in each of the epithelioid and sarcomatoid components of the mesotheliomas as follows: 3+, positive in >50% of tumor cells; 2+, positive in 25–50% of tumor cells; 1+, positive in 5–25% of tumor cells; and −, positive in <5% of tumor cells. In the evaluation of TLE1 immunohistochemistry, only intranuclear staining was considered to be positive. We also examined immunoreactivity of TLE1, β-catenin, and cyclin D1 in five cases of synovial sarcoma, all of which were confirmed to possess SS18–SSX fusion transcripts by reverse transcription-polymerase chain reaction [10], and nine non-neoplastic mesothelial lesions (seven organizing or fibrinous pleuritis, one mesothelial cyst, and one peritonitis due to appendicitis), and TLE1 in seven lung carcinomas (four adenocarcinomas, two sarcomatoid carcinomas, one squamous cell carcinoma). As negative controls, the reaction with non-immuned serum obtained from the normal mouse and the commercially available rabbit serum (Nichirei) was also examined with the heating antigen retrieval in citrate buffer or EDTA in specimens of malignant mesotheliomas. Correlation of the immunohistochemical expression between TLE1 and each marker examined was determined by Spearman’s rank correlation coefficient. Probability values of <0.05 were considered significant.
Results
The 29 patients with malignant mesotheliomas examined in this study consisted of 27 men and two women whose ages ranged from 51 to 85 years (mean, 63.3 years; median, 66 years). Of the 29 tumors, 27 tumors were located in the pleura, one in the peritoneum and one in the pericardium. Twenty-one patients had occupational history of asbestos exposure, although such clinical information was not available in two other cases. On the basis of a histological review, 12 (41.4%) tumors were subclassified as epithelioid type (Fig. 1a), ten (34.5%) as biphasic type, and seven (24%) as sarcomatoid type (Fig. 1e). The sarcomatoid components in ten biphasic mesotheliomas accounted for 20–80%. Information regarding the clinical outcome was available in 22 cases, and 20 patients died in 1–87 months (median, 7 months; mean, 14.2 months) after diagnosis. The other two patients were alive at 3 months after diagnosis.
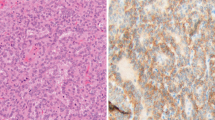
Hematoxylin & eosin staining (a, e, i) and immunohistochemical findings in epithelioid (case 7, a–d), and sarcomatoid (case 26, e–h) mesotheliomas and synovial sarcoma (i–l). TLE1 (b, f) and cyclin D1 (d, h) are expressed irrespective of the histologic subtypes of mesothelioma, whereas both subtypes lack nuclear expression of β-catenin (c, g). Many tumor cells in a synovial sarcoma are positively reactive to TLE1 (j), β-catenin (k), and cyclin D1 (l)
The immunohistochemical results of malignant mesotheliomas are shown in Table 1 and Fig. 1. TLE1 expression was variably observed in 28 of the 29 (96.6%) cases of malignant mesothelioma, displaying 2+ or 3+ positivity in 20 cases (69.0%), although the intensity of the immunoreaction was somewhat weaker than that in synovial sarcomas (Fig.1b, f, j). According to the histomorphological subtypes, eight of 12 epithelioid mesotheliomas and the epithelioid component of five of ten biphasic mesotheliomas [total, 13 of 22 (59.1%) mesotheliomas with epithelioid features] displayed 2+ or 3+ TLE1 positivity, whereas six of seven sarcomatoid mesotheliomas and the sarcomatoid component of six of ten biphasic mesotheliomas [total, 12 of 17 (70.6%) mesotheliomas with sarcomatoid features] showed 2+ or 3+ positivity. Only one tumor regarded as TLE1 negative was epithelioid mesothelioma. The expression of calretinin was seen in all 29 (100%) malignant mesotheliomas. HBME-1 was positive in 21 (72.4%) mesotheliomas, cytokeratin 5/6 in 20 (69.0%), and thrombomodulin in 24 (82.8%). The expression status of these markers was not correlated with TLE1 expression (Table 1). Nuclear β-catenin expression was confined to less than 5% of tumor cells in all the mesotheliomas, although its variable cytoplasmic and almost constant membranous expression was observed (Fig. 1c, g). Cyclin D1 was expressed in 26 (89.7%) mesotheliomas with 16 (55.2%) cases of 2+ or 3+ reactivity (Fig. 1d, h). TLE1 (3+, four cases; 2+, one case) and cyclin D1 (3+, two cases; 2+, one case; 1+, two cases) were immunoreactive in all five synovial sarcomas examined, and nuclear β-catenin expression was also variably recognized in four cases (3+, two cases; 2+, one case; 1+, one case; negative, one case) (Fig. 1i–l). Nuclei of non-neoplastic mesothelial cells were constantly positive for TLE1 (Fig. 2) but negative for β-catenin and cyclin D1. Among the seven lung carcinomas examined, six were negative for TLE1. The remaining one adenocarcinoma focally expressed TLE1.
Discussion
TLE1 is a member of the TLE gene family that encodes human transcriptional repressors, which bind to Tcf/Lef family transcription factors resulting in displacing β-catenin and repressing the Wnt/β-catenin signaling pathway [5]. The expression of TLE1 in synovial sarcomas has been demonstrated by several DNA microarray studies [11–14] and immunohistochemical studies [6–9, 15]. Because of its sensitivity and specificity, TLE1 was initially demonstrated to be a diagnostically useful immunohistochemical marker in synovial sarcoma [6]; however, Kosemehmetoglu et al. [9] showed that TLE1 expression is not specific for synovial sarcoma, being variably immunoreactive in 37% of soft tissue tumors other than synovial sarcoma. The TLE1-positive tumors included a malignant peripheral nerve sheath tumor, which may be one of the most important differential diagnoses of synovial sarcoma, solitary fibrous tumor, schwannoma, epithelioid sarcoma, acral myxoinflammatory fibroblastic sarcoma, chordoma, endometrial stromal sarcoma, leiomyosarcoma, liposarcoma, lipoma, myxofibrosarcoma, neurofibroma, rhabdomyosarcoma, undifferentiated pleomorphic sarcoma, Ewing sarcoma, gastrointestinal stromal tumor, chondrosarcoma, clear cell sarcoma, carcinosarcoma, fibroxanthoma, hemangiopericytoma, fibrosarcoma, and spindle epithelial tumor with thymus-like differentiation [6–9]. On the other hand, TLE1 expression is also seen in non-neoplastic mesothelial cells [9], but the expression of TLE1 in malignant mesotheliomas has not been fully evaluated [8]. It is sometimes difficult to distinguish malignant mesothelioma from synovial sarcoma originating in the thoracic cavity because they occasionally have overlapping clinicopathologic characteristics, including the biphasic epithelioid and sarcomatoid histomorphology, and expression of mesothelioma markers [2, 16]. The expression of calretinin, a calcium-binding protein expressed in both neural tissue and non-neural cell types, including mesothelium [17], has been recognized in not only most malignant mesotheliomas [4, 16, 17] but also in 60% of synovial sarcomas [4]. In addition, other markers sensitive to mesothelioma such as HBME-1, cytokeratin 5/6, and thrombomodulin are not specific for mesothelioma [4, 16] and may be expressed in a subset of synovial sarcoma [4]. In the present study, we examined the immunohistochemical expression of TLE1 in malignant mesotheliomas and compared its expression pattern with other markers that are typically known to be expressed in malignant mesotheliomas. In our results, 28 of 29 cases exhibited positive immunoreaction to TLE1, irrespective of their histological subtypes or of expression of other mesothelioma-related immunohistochemical markers. Despite the different detection systems (Envision system employing dextran polymer method or the Ventana automated immunostainer was utilized in the previous studies) and the different antigen retrieval methods used in the previous studies [6, 8, 9], the constant immunohistochemical expression of TLE1 in synovial sarcomas both in previous studies and ours suggest that these technical differences have not influenced the staining results. The difference of intensity of TLE1 expression between malignant mesotheliomas and synovial sarcoma was small and might be arbitrary. Immunoreactivity for TLE1 is by no means specific for synovial sarcoma and seems to have no use or, if any, only a supplementary role in differentiating synovial sarcoma from mesothelioma.
We also examined the immunohistochemical expression of β-catenin and cyclin D1, which may be associated with TLE1 via the Wnt signaling pathway, to assess the role of TLE1 in tumorigenesis or cell cycle regulation in malignant mesotheliomas. Our results demonstrated that most mesotheliomas exhibited a low or moderate level of nuclear expression of cyclin D1, whereas β-catenin was limited to membranous or cytoplasmic localization with no or quite limited nuclear accumulation [18]. In synovial sarcoma, SS18–SSX fusion protein has been shown to lead nuclear accumulation of β-catenin [14], and it has been speculated that TLE1 overexpression may represent a compensatory response to excessive β-catenin signaling or serve to limit transcriptional activation to certain genes, and that the nuclear expression of TLE1 and β-catenin resulting in transcriptional activation of target genes plays an important role in the pathogenesis of this tumor [6]. Previous studies showed that the SS18–SSX-mediated nuclear accumulation of cyclin D1 in synovial sarcomas was mediated by the PI3K-Akt-GSK3β pathway [19, 20] or aberrant β-catenin nuclear expression [21]. In general, cyclin D1 may also be activated without nuclear β-catenin overexpression via many different transcription signalings such as the MAPK pathway [22], JAK–STAT pathway [19, 23], and Notch–CSL pathway [20]. In malignant mesothelioma, MAPK is frequently expressed and activated with phosphorylation of the extracellular-regulated kinase, the c-Jun amino-terminal kinase, and p38 [24, 25]. The activation of cyclin D1 in malignant mesotheliomas seems to be induced via different mechanisms from synovial sarcoma and may be unrelated to TLE1 or the Wnt pathway. The constant TLE1 expression in non-neoplastic mesothelial cells suggests that TLE1 expression is not essential for malignant transformation of mesothelial cells. Further studies are required to clarify the exact role of TLE1 in these tumors.
In conclusion, our study showed that most of the malignant mesotheliomas expressed TLE1, and that immunohistochemistry for TLE1 is not useful or may have only a limited role in distinguishing between malignant mesothelioma and synovial sarcoma. The nuclear accumulation of β-catenin was not seen in malignant mesothelioma in contrast to synovial sarcoma, although both tumors frequently exhibited cyclin D1 overexpression, suggesting distinct biological significance of TLE1 in these tumors.
References
Churg A, Roggli V, Galateau-Salle F et al (2004) Mesothelioma. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris C (eds) Pathology and genetics of tumours of the lung, pleura, thymus and heart. IARC, Lyon, pp 128–136
Nicholson AG, Goldstraw P, Fisher C (1998) Synovial sarcoma of the pleura and its differentiation from other primary pleural tumours: a clinicopathological and immunohistochemical review of three cases. Histopathology 33:508–513
Okamoto S, Hisaoka M, Daa T, Hatakeyama K, Iwamasa T, Hashimoto H (2004) Primary pulmonary synovial sarcoma: a clinicopathologic, immunohistochemical, and molecular study of 11 cases. Hum Pathol 35:850–856
Miettinen M, Limon J, Niezabitowski A, Lasota J (2001) Calretinin and other mesothelioma markers in synovial sarcoma: analysis of antigenic similarities and differences with malignant mesothelioma. Am J Surg Pathol 25:610–617
Daniels DL, Weis WI (2005) β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12:364–371
Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, Downs-Kelly E, Corless CL, Rubin BP, van de Rijn M, Ladanyi M, Nielsen TO (2007) TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol 31:240–246
Folpe AL, Lloyd RV, Bacchi CE, Rosai J (2009) Spindle epithelial tumor with thymus-like differentiation: a morphologic, immunohistochemical, and molecular genetic study of 11 cases. Am J Surg Pathol 33:1179–1186
Jagdis A, Rubin BP, Tubbs RR, Pacheco M, Nielsen TO (2009) Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol 33:1743–1751
Kosemehmetoglu K, Vrana JA, Folpe AL (2009) TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 22:872–878
Tsuji S, Hisaoka M, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M, Nakamura T (1998) Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol 153:1807–1812
Baird K, Davis S, Antonescu CR, Harper UL, Walker RL, Chen Y, Glatfelter AA, Duray PH, Meltzer PS (2005) Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res 65:9226–9235
Beck AH, West RB, van de Rijn M (2010) Gene expression profiling for the investigation of soft tissue sarcoma pathogenesis and the identification of diagnostic, prognostic, and predictive biomarkers. Virchows Arch 456:141–151
Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO (2005) Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 18:68–74
Pretto D, Barco R, Rivera J, Neel N, Gustavson MD, Eid JE (2006) The synovial sarcoma translocation protein SYT-SSX2 recruits β-catenin to the nucleus and associates with it in an active complex. Oncogene 25:3661–3669
Knösel T, Heretsch S, Altendorf-Hofmann A, Richter P, Katenkamp K, Katenkamp D, Berndt A, Petersen I (2010) TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X;18): analysis of 319 cases. Eur J Cancer 46:1170–1176
Lucas DR, Pass HI, Madan SK, Adsay NV, Wali A, Tabaczka P, Lonardo F (2003) Sarcomatoid mesothelioma and its histological mimics: a comparative immunohistochemical study. Histopathology 42:270–279
Gotzos V, Vogt P, Celio MR (1996) The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol Res Pract 192:137–147
Abutaily AS, Collins JE, Roche WR (2003) Cadherins, catenins and APC in pleural malignant mesothelioma. J Pathol 201:355–362
Sakamoto K, Creamer BA, Triplett AA, Wagner KU (2007) The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol 21:1877–1892
Stahl M, Ge C, Shi S, Pestell RG, Stanley P (2006) Notch1-induced transformation of RKE-1 cells requires up-regulation of cyclin D1. Cancer Res 66:7562–7570
Horvai AE, Kramer MJ, O’Donnell R (2006) Beta-catenin nuclear expression correlates with cyclin D1 expression in primary and metastatic synovial sarcoma: a tissue microarray study. Arch Pathol Lab Med 130:792–798
Ortiz J, Funk C, Schäfer A, Lechner J (2009) Stu1 inversely regulates kinetochore capture and spindle stability. Genes Dev 23:2778–2791
Wang C, Li Z, Fu M, Bouras T, Pestell RG (2004) Signal transduction mediated by cyclin D1: from mitogens to cell proliferation: a molecular target with therapeutic potential. Cancer Treat Res 119:217–237
Sekido Y (2008) Molecular biology of malignant mesothelioma. Environ Health Prev Med 13:65–70
Vintman L, Nielsen S, Berner A, Reich R, Davidson B (2005) Mitogen-activated protein kinase expression and activation does not differentiate benign from malignant mesothelial cells. Cancer 103:2427–2433
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuyama, A., Hisaoka, M., Iwasaki, M. et al. TLE1 expression in malignant mesothelioma. Virchows Arch 457, 577–583 (2010). https://doi.org/10.1007/s00428-010-0975-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0975-8