Abstract
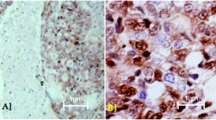
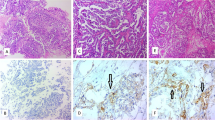
We have previously reported that alterations of p27Kip1-interacting cell-cycle proteins frequently occur during the development of endometriosis-associated ovarian clear cell adenocarcinoma (CCA; Yamamoto et al., Histopathology in press, 20). However, CCA also occurs in association with clear cell adenofibroma (CCAF). In this study, the expressions of p27Kip1-interacting proteins, i.e., p27Kip1, Skp2, Cks1, cyclin A, cyclin E, and the Ki-67 labeling index (LI), were analyzed in 25 CCAFs (11 benign and 14 borderline) and 15 CCAF-associated CCAs, and compared with the expression status of each protein in the 23 previously studied endometriosis-associated CCAs. Although aberrant expression of all p27Kip1-interacting proteins was more frequent in the CCAF-associated CCAs than in the benign CCAFs, statistical significance was found only for Cks1 overexpression. The frequencies of p27Kip1 downregulation and overexpression of Skp2 and cyclin A were significantly lower in CCAF-associated than in endometriosis-associated CCAs (P < 0.05, respectively). The frequencies of p27Kip1 downregulation and Skp2 overexpression in borderline CCAFs were significantly lower than those in atypical endometriosis components in endometriosis-associated CCAs (P < 0.05, respectively). Mean Ki-67 LI increased significantly through benign (4.9%) to borderline (11.1%) CCAF and to CCAF-associated CCA (30.6%), but the latter two values were significantly lower than those in atypical endometriosis (21.4%) and endometriosis-associated CCA (46.9%; P < 0.05, respectively). These data suggest that accumulated alterations of p27Kip1-interacting proteins may accelerate the development of CCAs regardless of their carcinogenetic pathways, but that tumor cells in the CCAF-associated pathway appear to show slower cell-cycle progression than those in the endometriosis-associated pathway, possibly accounting for the distinct clinicopathological features of the two CCA subtypes.



Similar content being viewed by others
References
Seidman JD, Russell P, Kurman RJ (2001) Surface epithelial tumors of the ovary. In: Kurman RJ (ed) Blaustein's pathology of the female genital tract, 5th edn. Springer-Verlag, New York, pp 791–904
Sugiyama T, Kamura T, Kigawa J et al (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589
Ikeda K, Sakai K, Yamamoto R et al (2003) Multivariate analysis for prognostic significance of histologic subtype, GST-pi, MDR-1, and p53 in stages II-IV ovarian cancer. Int J Gynecol Cancer 13:776–784
Tavassoli FA, Devilee P (eds) (2003) World Health Organization classification of tumours. Pathology and genetics of tumours of the breast and female genital organs. IARC Press, Lyon
Kaku T, Ogawa S, Kawano Y et al (2003) Histological classification of ovarian cancer. Med Electron Microsc 36:9–17
Gynecologic cancer committee, Japan society of obstetrics and gynecology (2008) Annual report of gynecological cancer patients in Japan 2006. Acta Obstet Gynaecol Jpn 60:1001–1085 in Japanese
Takano M, Sugiyama T, Yaegashi N et al (2008) Low response rate of second-line chemotherapy for recurrent or refractory clear cell carcinoma of the ovary: a retrospective Japan Clear Cell Carcinoma Study. Int J Gynecol Cancer 18:937–942
Takano M, Kikuchi Y, Yaegashi N et al (2006) Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer 94:1369–1374
Sampson JA (1925) Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Arch Surg 10:1–72
Vercellini P, Parazzini F, Bolis G et al (1993) Endometriosis and ovarian cancer. Am J Obstet Gynecol 169:181–182
Fukunaga M, Nomura K, Ishikawa E et al (1997) Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology 30:249–255
Ogawa S, Kaku T, Amada S et al (2000) Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol 77:298–304
LaGrenade A, Silverberg SG (1988) Ovarian tumors associated with atypical endometriosis. Hum Pathol 19:1080–1084
Mostoufizadeh M, Scully RE (1980) Malignant tumors arising in endometriosis. Clin Obstet Gynecol 23:951–963
Seidman JD (1996) Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol 15:1–9
Varma R, Rollason T, Gupta JK et al (2004) Endometriosis and the neoplastic process. Reproduction 127:293–304
Jiang X, Hitchcock A, Bryan EJ et al (1996) Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumor suppressor gene loci. Cancer Res 56:3534–3539
Jiang X, Morland SJ, Hitchcock A et al (1998) Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res 58:1707–1712
Sato N, Tsunoda H, Nishida M et al (2000) Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res 60:7052–7056
Yamamoto S, Tsuda H, Miyai K, et al (2009) Cumulative alterations of p27Kip1-related cell cycle regulators in the development of endometriosis-associated ovarian clear cell adenocarcinoma. Histopathology (in press)
Bell DA, Scully RE (1985) Benign and borderline clear cell adenofibromas of the ovary. Cancer 56:2922–2931
Roth LM, Langley FA, Fox H et al (1984) Ovarian clear cell adenofibromatous tumors. Benign, of low malignant potential, and associated with invasive clear cell carcinoma. Cancer 53:1156–1163
Yamamoto S, Tsuda H, Yoshikawa T et al (2007) Clear cell adenocarcinoma associated with clear cell adenofibromatous components: a subgroup of ovarian clear cell adenocarcinoma with distinct clinicopathologic characteristics. Am J Surg Pathol 31:999–1006
Yamamoto S, Tsuda H, Takano M et al (2008) Clear-cell adenofibroma can be a clonal precursor for clear-cell adenocarcinoma of the ovary: a possible alternative ovarian clear-cell carcinogenic pathway. J Pathol 216:103–110
Yamamoto S, Tsuda H, Takano M et al (2008) Expression of platelet-derived growth factors and their receptors in ovarian clear-cell carcinoma and its putative precursors. Mod Pathol 21:115–124
Filipits M, Puhalla H, Wrba F (2003) Low p27Kip1 expression is an independent prognostic factor in gallbladder carcinoma. Anticancer Res 23:675–679
Hui AM, Li X, Shi YZ, Torzilli G, Takayama T, Makuuchi M (2000) p27(Kip1) expression in normal epithelia, precancerous lesions, and carcinomas of the gallbladder: association with cancer progression and prognosis. Hepatology 31:1068–1072
Huang HY, Kang HY, Li CF et al (2006) Skp2 overexpression is highly representative of intrinsic biological aggressiveness and independently associated with poor prognosis in primary localized myxofibrosarcomas. Clin Cancer Res 12:487–498
Li SH, Li CF, Sung MT et al (2007) Skp2 is an independent prognosticator of gallbladder carcinoma among p27(Kip1)-interacting cell cycle regulators: an immunohistochemical study of 62 cases by tissue microarray. Mod Pathol 20:497–507
Zhang H, Kobayashi R, Galaktionov K, Beach D (1995) p19skp1 and p45skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 82:915–925
Gstaiger M, Jordan R, Lim M et al (2001) Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA 98:5043–5048
Chao Y, Shih Y-L, Chiu J-H et al (1998) Overexpression of cyclin A but not Skp2 correlated with the tumor relapse of human hepatocellular carcinoma. Cancer Res 58:985–990
Latres E, Chiarle R, Schulman BA et al (2001) Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA 98:2515–2520
Shigemasa K, Gu L, O'Brien TJ, Ohama K (2003) Skp2 expression is a prognostic factor in patients with ovarian adenocarcinoma. Clin Cancer Res 9:1756–1763
Sui L, Dong Y, Watanabe Y et al (2006) Clinical significance of Skp2 expression, alone and combined with Jab1 and p27 in epithelial ovarian tumors. Oncol Rep 15:765–771
Itamochi H, Kigawa J, Sugiyama T et al (2002) Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet Gynecol 100:281–287
Shimizu M, Nikaido T, Toki T, Shiozawa T, Fujii S (1998) Clear cell carcinoma has an expression pattern of cell cycle regulatory molecules that is unique among ovarian adenocarcinomas. Cancer 85:669–677
Hwang HC, Clurman BE (2005) Cyclin E in normal and neoplastic cell cycles. Oncogene 24:2776–2786
Nakayama KI, Hatakeyama S, Nakayama K (2001) Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem Biophys Res Commun 282:853–860
Anttila MA, Kosma V-M, Hongxiu J et al (1999) p21/WAF1 expression as related to p53, cell proliferation and prognosis in epithelial ovarian cancer. Br J Cancer 79:1870–1878
Acknowledgments
This work was supported in part by a grant-in-aid for promotion of defense medicine from the Ministry of Defense, Japan (S.Y., H.T., and O.M.), and by a grant-in-aid for cancer research from the Ministry of Health, Labour, and Welfare, Japan (H.T.). The authors are thankful to Ms. Kozue Suzuki for technical assistance with immunohistochemistry.
Conflict of interest statement
The authors declare no actual or potential conflicts of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, S., Tsuda, H., Miyai, K. et al. Aberrant expression of p27Kip1-interacting cell-cycle regulatory proteins in ovarian clear cell carcinomas and their precursors with special consideration of two distinct multistage clear cell carcinogenetic pathways. Virchows Arch 455, 413–422 (2009). https://doi.org/10.1007/s00428-009-0844-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-009-0844-5