Abstract
Background
Ovarian cancer is the most common gynecological malignancy. In patients with advanced ovarian cancer, some biological parameters have prognostic implementations. P27kip1 is an inhibitor of a cycline-dependent kinase, its loss, can contribute to tumor progression.
Objective
This study aimed to examine the importance of P27KIP1 protein in predicting the prognosis and response to neoadjuvant chemotherapy in patients with advanced ovarian epithelial cancer and to compare the outcomes of immunohistochemistry with Quantitative Real-time PCR.
Patients and methods
We have studied P27KIP1expression by both immunohistochemistry and Quantitative Real-time PCR from 88 patients with advanced ovarian carcinomas undergone radical debulking surgery and received Paclitaxel followed by Cisplatin every 3 weeks for a total of 6 cycles. We also studied their association with both chemotherapy response and patient survival.
Results
Nuclear expression of p27KIP1 protein was intense in 86 normal ovarian tissues and 42 of 88 carcinomas. The P27kip1mRNA expression level by qRT-PCR was very low in ovarian cancer tissues relative to its adjacent normal tissues. The results were statistically significant by both methods of determination. p27KIP1 expression was significantly related to good prognostic parameters as low stage tumors, differentiated tumors, absence of ascites, residual disease < 2 cm, and response to chemotherapy but not with histopathological type in case of determination by immunohistochemistry. Comparison of P27kip1 by both immunohistochemistry and qRT-PCR with different prognostic parameters revealed no significant difference between both methods in the assessment of these parameters. In 4 years of follow-up, 20.5% of patients were alive without evidence of disease. 6.8% were alive with disease. The disease-related four -year survival rate for the whole group was 28.2%. In multivariate analysis, residual disease, histological type, tumor differentiation, ascites was of independent prognostic significance.
Conclusion
In ovarian cancer, patients with loss of p27KIP1 expression are at a greater likelihood of disease progression, p27KIP1 may be used as a molecular marker to predict response to chemotherapy and prognosis. Both immunohistochemistry and qRT-PCR have equal reliability in the determination of p27 KIP1.
Similar content being viewed by others
Introduction
Epithelial ovarian cancer is the most fatal gynecological malignancy in developing countries [1]. Approximately 80% of patients are diagnosed with an advanced stage [2], which is associated with a 5-year survival rate of only 30%. This, despite improvements in long-term survival, obtained through the use of combination chemotherapy, mainly with cisplatin and lately with paclitaxel [3]. Several factors attribute to the bad prognosis of patients with an advanced stage ovarian carcinoma, including the inability to perform complete cytoreductive surgery to eradicate the metastatic disease in > 75% of patients, underlying resistant to chemotherapy in over half of these patients and the emergence of chemoresistance in approximately half of the originally sensitive patients [4]. Although clinical-pathological characteristics of ovarian cancer other than the stage of the disease, such as residual disease size following debulking surgery, histological grade and type, lymph node status and the existence of ascites are additionally of proven prognostic value, individual patients may show considerable chemosensitivity variations although they share the same clinical-pathological characteristics [5]. The pathogenesis of most human cancers involved dysregulation of normal cell cycle control. Abnormal expressions of regulatory proteins that regulate G1-S phase transition are frequently noticed as a critical, rate-limiting step in the progression of the cell cycle. G1-S transformation involves phosphorylation of the retinoblastoma protein pRb, leading to the production of transcription factors in the E2F family, which activate genes necessary for entry into the S phase [6]. PRb phosphorylation is launched by complexes of cyclin D1/(CDK)4–6 and ended in late G1 by cyclin E/CDK2. Changes in the expression of cyclin and/or cycline-dependent kinase (CDK) lead to enhanced cell proliferation and are undoubtedly believed to contribute to malignancy. Also, down-regulation or inactivation of CDK inhibitors, including p21Waf1/Cip1, p27Kip1, and p16Ink4a, usually causing G1 arrest by binding to cyclin-CDK complexes, is frequently found in various human tumors, making sufficiently the cell vulnerable to uncontrolled extracellular proliferation signals further [7]. Several trials have found expression in epithelial ovarian cancer (EOC) of these critical cell cycle regulatory proteins. Despite frequent detection of alterations in expression levels, there are many conflicting results, making it hard to delineate the role of individual genes in the development and progression of EOC [8]. Several trials have shown that p27KIP1 is a promising molecular marker of the poor prognosis in several cancers [9,10,11,12,13]. Several trials have detected an expression in epithelial ovarian cancer (EOC) of this critical cell cycle regulatory protein. Despite frequent detection of alterations in expression levels, there are many conflicting results, making it hard to carefully delineate the role of particular genes in the potential development and progression of EOC [8]. Several trials have sufficiently shown that p27KIP1 is properly a promising molecular marker of the poor prognosis in several cancers [9,10,11,12,13].
Patients and methods
Patients
This study comprises 88 patients with the International Gynecology and Obstetrics Federation (FIGO) [14] stage III epithelial cancer ovary confirmed histopathologically between May 2012 and April 2019. All patients underwent initial debulking surgery within 6 weeks before chemotherapy. Written informed consent has been obtained from all cases included. The minimum age for active recruitment in the study was at least 18 years of age. All patients had a normal blood picture, renal and hepatic function at the entry of the study. The study was approved by the Committee of Ethics of research, Zagazig University. Informed consent was obtained from all participating patients before enrollment in the study.
Exclusion criteria included patients with ovarian low malignant potential tumors; poor performance status of over 2 Eastern Cooperative Oncology Groups (ECOG) [15]; estimated glomerular filtration rate (GFR) of less than 60 mL/minute; severe neuropathy; prior chemotherapy or radiotherapy for ovarian cancer and congestive heart failure or arrhythmias.
Management
Postoperative chemotherapy comprised Paclitaxel 175 mg/m2 IV over 3 h followed by Cisplatin 75 mg/m2 IV infusion after vigorous hydration was typically administered every 3 weeks for 6 cycles for all patients included in the study. Gynecological examination, abdominopelvic ultrasonography, CA-125 assay were performed monthly. Other radiological studies, such as CT or MRI of the abdomen and pelvis, were performed before chemotherapy and every two-month if typically needed for Clinical response evaluation according to the revised RECIST (Response Evaluation Criteria in Solid Tumors) criteria [16].
Four weeks after the 6th cycle of chemotherapy, patients with a clinical complete response underwent a laparoscopy. In laparoscopy negative cases, a laparotomy was performed to evaluate the pathological response by multiple biopsies. After pathological assessment of biopsies, patients were properly assigned to one of three groups: complete response, partial response with the microscopic disease only, and persistent disease.
Immunohistochemistry
Immunohistochemistry was performed as mentioned previously [17]. Briefly, Six-micron sections were cut from formalin-fixed paraffin-embedded tissue blocks, deparaffinized in xylene, and rehydrated. For antigen retrieval and detection of p27KIP1, the sections were heated in a microwave oven for a total of 30 min (three cycles of 10 min each) in 10 mmol/L sodium citrate buffer at pH 6.0. Endogenous peroxidase activity was eliminated by preincubation in 2% H2O2 in methanol for 30 min followed by three washes in phosphate-buffered saline (PBS). The sections were stained using standard streptavidin-biotin complex immunoperoxidase methods (Santa Cruz Biotechnology, Santa Cruz, CA) on a Ventana ES machine (Ventana Medical Systems, Tucson, AZ). The primary antibodies for p27KIP1 were NCL-P27 monoclonal antibody (1:40). All antibodies were diluted in PBS containing 1% normal rabbit serum. Peroxidase activity was localized with chromogen 3,3′-diaminobenzidine tetrachloride in 0.5 mmol/L Tris buffer. The slides were counterstained with Delafield’s hematoxylin. Normal ovarian tissue served as a positive control for p27KIP1 immunostaining.
p27Kip1 scoring
Samples were coded and the percentage of immunostained cells was assessed. Expression was categorized as positive (staining in ≥5% of cells) or negative (staining in < 5% of cells) as described previously. At least 20 high-power fields were selected randomly and 2000 cells were counted [18].
Quantitative real-time polymerase chain reaction
RNA extraction
RNA extraction The extraction of total RNA from tissue samples (normal and cancerous ovarian tissue) was performed using the RNase Kit (Qiagen, Germany). The purity and concentration of RNA were verified by evaluating the optical density (OD) at 260 and 280 nm using a spectrophotometer with an acceptable A260/A280 ratio of 1.8 to 2.1.
Quantitative real time-PCR of P27kip1 mRNA gene expression
One microgram of RNA was transcribed reversely using the (QuantiTect Reverse Transcription Kit) as instructed by the manufacturer. P27kip1 expression was evaluated by quantitative real-time PCR (qRT-PCR) using the following primers: P27 kip1 forward primer: GGCTTTCAGATTCCCAACTT and P27 kip1 reverse primer: AGCCTCCCCACTCTCGTCT and ABL forward primer: AGTCTCAGGATGCAGGTGCT and ABL reverse primer: TAGGCTGGGGCTTTTTGTAA as ABL has been regarded to be a housekeeping gene.
PCR amplification was conducted in 25 μl of 12.5 μl 2x QuantiFast SYBR Green PCR Master Mix, 1 μM of each primer and 2 μl of cDNA under the following circumstances:
Thermal cycling conditions for each reaction included an initial hold at ninety-five °C for ten minutes, followed by forty denaturation cycles at ninety-five °C for ten seconds and annealing/extension at sixty °C for thirty seconds. Fold change in the expression of mRNA of cancerous and normal control tissue samples was obtained using the Livak method. P27kip1expression level was determined by Stratagene, MX3000P Quantitative PCR System (Agilent Technologies), and evaluated using MxPro QPCR Software (Agilent Technologies). The kit was provided by QIAGEN, Valencia, CA, USA. The 2−ΔΔCT method [19] was used to calculate the relative level of gene expression compared to the β-actin housekeeping control.
Statistical analysis
Analysis of the data was implemented using the SPSS Statistics 16.0 (SPSS Inc., Chicago, IL, USA) and Graph Pad Prism 6.07 software (La Jolla, CA, USA).
Results
P27kip1 expression
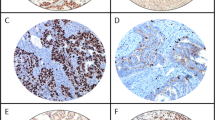
P27kip1 has been analyzed using immunohistochemistry and quantitative real-time PCR. Immunoreactivity for P27KIP1 antigens has shown nuclear staining and weak cytoplasmic staining. Nuclear expression of p27KIP1 protein was intense in 86 normal adjacent ovarian tissues (NAOT) and in 42 of 88 carcinomas (ECO) (47.7%), the difference between both groups was statistically significant (t = 6.811, p < 0.001) (Fig. 1 and Fig. 2 a).
The P27kip1mRNA expression level by qRT-PCR in malignant ovarian tissues ranged between 0.001and 1.914 with a mean ± SD level of 0.743 ± 0.54 while in the normal adjacent ovarian tissues, the mRNA ranged between 5.6 and 15.35 with a mean ± SD level of 10.098 ± 2.716. The difference between both groups was statistically significant (t = 31.682, p < 0.001) (Fig. 2b).
P27kip1, clinical, and pathological characteristics
In the present study, the mean age of the patients was 44 ± 10.3 years (range 21–66 years). Sixty-six (75%) patients had an initial stage IIIC; 58 (65.9%) had moderate or poorly differentiated tumors. Serous carcinomas were the most prevalent pathological type (75%) and ascites were present in 60 (68.2%) patients. After initial debulking surgery, 56 patients (63.6%) had a residual disease greater than 2 cm in size. After chemotherapy, forty-six patients (52.3%) achieved a treatment response (Table 1).
P27kip1was studied with the clinical and pathological data of the patients. According to P27kip1 immunoreactivity, we observed a statistically significant relation between P27kip1 expression and non-expression by immunohistochemistry and FIGO stage, tumor grade, the presence or absence of ascites, residual tumors after surgery and response to chemotherapy. No difference was observed between the serous and non-serous pathological types (Table 1). When analyzing the clinical and pathological data with P27kip1 upregulation and downregulation by qRT-PCR, we found a statistically significant difference between all clinical and pathological data and up or down P27kip1 regulation (Table 1).
P27kip1 and prognostic parameters
Expression of P27kip1by immunohistochemistry was associated with good prognostic parameters as low stage tumors (P = 0.040), differentiated tumors (P < 0.001), absence of ascites (P < 0.001), residual disease < 2 cm (P < 0.001) and response to chemotherapy (P = 0.004) but was uncorrelatedwith the histopathological type (Table 1). Measurement of P27kip1 mRNA by qRT-PCR revealed statistically significant upregulation with all prognostic parameters as analysis with immunohistochemistry but including the histopathological type (Table 1).
Statistical evaluation of the patients with positive P27 immunostaining and upregulation by RT-PCR with each of the clinical and pathological parameters revealed no significant difference between both techniques and all parameters (Table 2). Meanwhile, analysis of the patients according to their P27 expression or non-expression by IHC and according to up or downregulation by RT-PCR and each of clinical and pathological parameters revealed a significant correlation with each parameter (Table 2).
Comparison of P27KIP1 expression by both immunohistochemistry and qRT-PCR with each subcategory of different prognostic parameters revealed no significant difference between both methods in the assessment of these parameters (Table 3).
P27 KIP1 and patients survival
During 4 years of follow-up, 18 patients (20.5%) were alive without evidence of disease; six were alive with disease (6.8%), whereas 64 had died of ovarian cancer (72.7%). The disease-related 4-year survival rate for the whole group was 28.2%. The median progression-free survival time was 11 months, and the median corrected survival time was 21 months. In univariate analysis, FIGO stage (P = 0.04), histological type (P = 0.094), differentiation grade (P < 0 .001), ascites (P < 0.001), residual disease (P < 0.001) and response to chemotherapy (P = 0.004) were correlated to corrected survival. Expression p27 KIP1 by immunostaining or qRT-PCR did not reach statistical significance when its effect on survival was examined. Meanwhile, in multivariate analysis, residual disease, histological type, differentiation, ascites was of independent prognostic significance (Table 4).
Discussion
In this study, the prognostic role of p27kip1 expression was evaluated in 88 patients with locally advanced ovarian cancer. P27kip1 was regarded as a tumor suppressor gene and the loss of its function was associated with the development of many kinds of human cancer. The tumor suppressor function of p27kip1 was first involved in the regulation of the cell cycle [20]. Several trials have shown that expression of the CDK inhibitor p27 KIP1 is a good predictor of longer time to progression and overall survival in many ovarian cancer patients [21,22,23,24]. Other studies evaluating the prognostic function of p27 KIP1 expression in various types of tumors. In specific, loss of p27 KIP1 expression markedly improves the risk of recurrence and death related cancer in breast [25], prostate [26], bladder [27], hepatocellular [28], and colorectal [29] carcinomas. Although p27 expression appears to be an important predictor of clinical behavior in several malignancies, current evidence suggests that loss of p27Kip1 protein is not attributable to structural alterations of the gene [20] but may result from increased degradation of the protein-mediated by ubiquitin-proteasome pathway [29, 30].
In the present study, correlations were observed with favorable prognostic factors such as lower FIGO stage, differentiated tumors, absence of ascites and residual disease < 2 cm and response to chemotherapy. p27 KIP1 was shown not to be of prognostic significance in advanced ovarian cancer by both immunostainings and measurement of mRNA by real-time PCR. This is comparable with the results of Mudan Lu et al., that discovered that p27 KIP1 expression by immunohistochemistry had independent prognostic significance in the meta-analysis, including nine trials: six conducted in Europe, three conducted in the USA, and one study conducted in Asia [31]. Hafez et al. [32] also discovered the same outcomes, by evaluating the concentrations of gene expression using real-time PCR and Western blotting.
We also examined the possible predictive value of p27 KIP1 in the prediction of response to chemotherapy, as there is increasing evidence that p27 KIP1 functions as a regulator of drug resistance in solid tumors [30]. In human colon cancer cells, overexpression of p27KIP1 was linked to increased resistance to drugs like cisplatin, doxorubicin, and etoposide [30], these drugs also used in the treatment of ovarian cancer. In our study, a significant correlation was found between the level of p27KIP1 expression and response to chemotherapy, as its decreased expression was linked to no or poor response to chemotherapy. Mudan Lu et al. [31] in their meta-analysis also observed that p27KIP1-positive cases have a higher response to chemotherapy, especially in patients who were optimally cytoreduced at first surgery. Platinum-based chemotherapy induces apoptosis in tumor cells, and reduced susceptibility to apoptosis has been proposed as a major mechanism responsible for chemotherapy resistance [33]. Recent data also suggest that p27KIP1 overexpression induces apoptosis in many types of cancers through a p53-independent pathway [34,35,36,37]. Additionally, in many human breast cancer specimens, p27KIP1 levels showed a significant correlation with the apoptotic index and predictive value for the benefit of chemotherapy [34].
P27KIP1 expression may confer possible p53-independent apoptosis sensitivity, thus increasing the sensitivity of ovarian cancer cells to chemotherapy agents.
Conclusion
This study provides evidence about the role of p27 KIP1 in ovarian cancer patients as a predictor for patient outcomes. Patients with ovarian cancer who have a loss of p27KIP1 expression are at a greater likelihood of disease progression and may eventually benefit from more aggressive therapy. The reliability of p27 KIP1 as a possible marker in the clinical routine evaluation and management of ovarian cancer merits further analysis in a study involving a large number of patients. Finally, this study provides evidence of equal reliability of immunohistochemistry and qRT-PCR in the determination of p27 KIP1.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
References
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–96.
Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016;1:CD005340.
Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–36. https://doi.org/10.1016/S1470-2045(15)00086-8.
Kartal-Yandim M, Aysun AG, Yusuf B. Molecular mechanisms of drug resistance and its reversal in cancer. Crit Rev Biotechnol. 2015;36(4):716–26. https://doi.org/10.3109/07388551.1015957.
Dahm-Kähler P, Borgfeldt C, Holmberg E, Staf C, Falconer H, Bjurberg M, et al. Population-based study of survival for women with serous cancer of the ovary, fallopian tube, peritoneum or undesignated origin-on behalf of the Swedish gynecological cancer group (SweGCG). Gynecol Oncol. 2017;144(1):167–73. https://doi.org/10.1016/j.ygyno.2016.10.039.
Thwaites MJ, Cecchini MJ, Passos DT, Welch I, Dick FA. Interchangeable roles for E2F transcriptional repression by the retinoblastoma protein and p27KIP1–cyclin-dependent kinase regulation in cell cycle control and tumor suppression. Mol Cell Biol. 2017;37(2):e00561–16. https://doi.org/10.1128/MCB.00561-16.
Mazumdar A, Hill J, Zhang Y, Bollu LR, Tsimelzon A, Chang J, Mills G, Brown P. Induced expression of PPM1A in ER-negative breast cancer cells inhibits growth by suppressing CDK phosphorylation. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1–5; Washington, DC. Philadelphia (PA): AACR; Cancer Res 2017; 77(13 Suppl):Abstract nr 5525. doi:https://doi.org/10.1158/1538-7445.AM2017-5525.
Grassi ML, de Souza PC, Thomé CH, Lanfredi GP, Poersch A, Faça VM. Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. J Proteomics. 2017;151:2–11. https://doi.org/10.1016/j.jprot.2016.06.009.
Bakr MM, Guan S, Firth N, Love RM. Cyclin D1 and P27KIP1: the gatekeepers of dysplasia. J Immunological Sci. 2018;2(3):30–9.
Gao Y, Shen J, Choy E, Mankin H, Hornicek F, Duan Z. Inhibition of CDK4 sensitizes multidrug resistant ovarian cancer cells to paclitaxel by increasing apoptosiss. Cell Oncol. 2017;40(3):209–18. https://doi.org/10.1007/s13402-017-0316-x.
Lu M, Wang Y, Xu F, Xiang J, Chen D. The prognostic of p27 kip1 in ovarian cancer: a meta-analysis. Arch Gynecol Obstet. 2016;293(1):169–76. https://doi.org/10.1007/s00404-015-3817-8.
Lu M, Wang Y, Xu F, Xiang J, Chen D. The prognostic of p27 kip1 in ovarian cancer: a meta-analysis. Arch Gynecol Obstet. 2016;293(1):169–76. https://doi.org/10.12892/ejgo3784.2018.
Al-Maghrabi J, Emam E, Gomaa W, Saggaf M, Buhmeida A, Al-Qahtani M, et al. C-MET immunostaining in colorectal carcinoma is associated with local disease recurrence. BMC Cancer. 2015;15(1):676. https://doi.org/10.1186/s12885-015-1662-6.
Prat J. FIGO committee on gynecologic oncology. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26(2):87–9. https://doi.org/10.3802/jgo.2015.26.2.87.
Prigerson HG, Bao Y, Shah MA, Paulk ME, LeBlanc TW, Schneider BJ, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1(6):778–84. https://doi.org/10.1001/jamaoncol.2015.2378.
Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. Am J Roentgenol. 2010;195(2):281–9.
Guo Y, Sklar GN, Borkowski A, Kyprianou N. Loss of the cyclin-dependent kinase inhibitor p27 (Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res. 1997;3(12):2269–74.
Masciullo V, Sgambato A, Pacilio C, Pucci B, Ferrandina G, Palazzo J, et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res. 1999;59(15):3790–4.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3(6):1101.
Kalra S, Joshi G, Munshi A, Kumar R. Structural insights of cyclin dependent kinases: implications in design of selective inhibitors. Eur J Med Chem. 2017;142:424–58. https://doi.org/10.1016/j.ejmech.2017.08.071.
Masciullo V, Ferrandina G, Pucci B, Fanfani F, Lovergine S, Palazzo J, et al. p27Kip1 expression is associated with clinical outcome in advanced epithelial ovarian cancer: multivariate analysis. Clin Cancer Res. 2000;6(12):4816–22.
Hashimoto T, Yanaihara N, Okamoto A, Nikaido T, Saito M, Takakura S, et al. Cyclin D1 predicts the prognosis of advanced serous ovarian cancer. Exp Ther Med. 2011;2(2):213–9. https://doi.org/10.3892/etm.2011.194.
Psyrri A, Bamias A, Yu Z, Weinberger PM, Kassar M, Markakis S, et al. Subcellular localization and protein levels of cyclin-dependent kinase inhibitor p27 independently predict for survival in epithelial ovarian cancer. Clin Cancer Res. 2005;11(23):8384–90.
Bali A, O’Brien PM, Edwards LS, Sutherland RL, Hacker NF, Henshall SM. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004;10(15):5168–77.
Kalra S, Joshi G, Munshi A, Kumar R. Structural insights of cyclin dependent kinases: implications in design of selective inhibitors. Eur J Med Chem. 2017;142:424–58.
Cusan M, Mungo G, De Marco ZM, Segatto I, Belletti B, Baldassarre G. Landscape of CDKN1B mutations in luminal breast cancer and other hormone-driven human tumors. Front Endocrinol. 2018;9:393. https://doi.org/10.3389/fendo.2018.00393.
Ronen S, Abbott DW, Kravtsov O, Abdelkader A, Xu Y, Banerjee A, et al. PTEN loss and p27 loss differ among morphologic patterns of prostate cancer, including cribriform. Hum Pathol. 2017;65:85–91. https://doi.org/10.1016/j.humpath.2017.04.024.
Hu B, Hua L, Ni W, Wu M, Yan D, Chen Y, et al. Nucleostemin/GNL3 promotes nucleolar polyubiquitylation of p27kip1 to drive hepatocellular carcinoma progression. Cancer Lett. 2017;338:220–9. https://doi.org/10.1016/j.canlet.2016.12.008.
Li W, Zhang G, Wang HL, Wang L. Analysis of expression of cyclin E, p27kip1 and Ki67 protein in colorectal cancer tissues and its value for diagnosis, treatment and prognosis of disease. Eur Rev Med Pharmacol Sci. 2016;20(23):4874–9.
Randles L, Anchoori RK, Roden RB, Walters KJ. The proteasome ubiquitin receptor hRpn13 and its interacting deubiquitinating enzyme Uch37 are required for proper cell cycle progression. J Biol Chem. 2016;291(16):8773–83. https://doi.org/10.1074/jbc.%20M115.694588.
Lu M, Wang Y, Xu F, Xiang J, Chen D. The prognostic of p27kip1 in ovarian cancer: a meta-analysis. Arch Gynecol Obstet. 2016;293:169. https://doi.org/10.1007/s00404-015-3817-8.
Hafez MM, Alhoshani AR, Al-Hosaini KA, Alsharari SD, Al Rejaie SS, Sayed-Ahmed MM, et al. SKP2/P27Kip1 pathway is associated with advanced ovarian cancer in Saudi patients. Asian Pac J Cancer Prev. 2015;16(14):5807–15.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46. https://doi.org/10.1038/nrd4504.
Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93.
Lu K, Wang J, Song Y, Zhao S, Liu H, Tang D, et al. miRNA-24-3p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting p27Kip1. Oncol Rep. 2015;34(2):995–1002. https://doi.org/10.3892/or.2015.4025.
Liu H, Liu Y, Bian Z, Zhang J, Zhang R, Chen X, et al. Circular RNA YAP1 inhibits the proliferation and invasion of gastric cancer cells by regulating the miR-367-5p/p27 Kip1 axis. Mol Cancer. 2018;17(1):151.
Ramu V, Gill MR, Jarman PJ, Turton D, Thomas JA, Das A, et al. A cytostatic ruthenium (II)–platinum (II) Bis (terpyridyl) anticancer complex that blocks entry into S phase by up-regulating p27KIP1. Chem A Eur J. 2015;21:9185–97. https://doi.org/10.1002/chem.201500561.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Amani A Alrehaili performed the research and analyzed the data. M AlMourgi: performed the research and analyzed the data. Amal F Gharib: designed the research study; performed the research; analyzed the data and wrote the paper. W H Elsawy: designed the research study; performed the research; analyzed the data and wrote the paper. Khadiga Ahmed Ismail: performed the research and analyzed the data. Howaida Mahmoud Hagag: performed the research, analyzed the data and contributed essential reagents. Farah Anjum: analyzed the data. Nermin Raafat: performed the research, analyzed the data and contributed essential reagents. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Committee of Ethics of research, Zagazig University. Informed consent was obtained from all participating patients before enrollment in the study.
Consent for publication
All authors consent to the publication of the manuscript in ACR, should the article be accepted by the Editor-in-chief upon completion of the refereeing process.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alrehaili, A.A., AlMourgi, M., Gharib, A.F. et al. Clinical significance of p27 Kip1 expression in advanced ovarian cancer. Appl Cancer Res 40, 6 (2020). https://doi.org/10.1186/s41241-020-00090-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41241-020-00090-1