Abstract
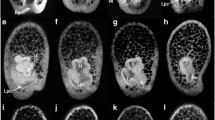
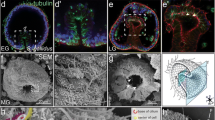
Echinoderms are unique among bilaterians for their derived, nonbilateral adult body plan. Their radial symmetry emerges from the bilateral larval body plan by the establishment of a new axis, the adult oral–aboral axis, involving local mesoderm–ectoderm interactions. We examine the mechanisms underlying this transition in the direct-developing sea urchin Heliocidaris erythrogramma. Adult ectoderm arises from vestibular ectoderm in the left vegetal quadrant. Inductive signals from the left coelom are required for adult ectodermal development but not for initial vestibule formation. We surgically removed gastrula archenteron, making whole-ectoderm explants, left-, right-, and animal-half ectoderm explants, and recombinants of these explants with left coelom. Vestibule formation was analyzed morphologically and with radioactive in situ hybridization with HeET-1, an ectodermal marker. Whole ectodermal explants in the absence of coelom developed vestibules on the left side or ventrally but not on the right side, indicating that left–right polarity is ectoderm autonomous by the gastrula stage. However, right-half ectodermal explants robustly formed vestibules that went on to form adult structures when recombined with the left coelom, indicating that the right side retains vestibule-forming potential that is normally suppressed by signals from the left-side ectoderm. Animal-half explants formed vestibules only about half the time, demonstrating that animal–vegetal axis determination occurs earlier. However, when combined with the left coelom, animal-half ectoderm always formed a vestibule, indicating that the left coelom can induce vestibule formation. This suggests that although coelomic signals are not required for vestibule formation, they may play a role in coordinating the coelom–vestibule interaction that establishes the adult oral–aboral axis.





Similar content being viewed by others
References
Aihara M, Amemiya S (2001) Left–right positioning of the adult rudiment in sea urchin larvae is directed by the right side. Development 128:4935–4948
Angerer LM, Angerer RC (1991) Localization of mRNAs by in situ hybridization. Methods Cell Biol 35:37–71
Angerer LM, Angerer RC (2003) Patterning the sea urchin embryo: gene regulatory networks, signaling pathways, and cellular interactions. Curr Top Dev Biol 53:159–198
Brandhorst BP, Klein WH (2002) Molecular patterning along the sea urchin animal–vegetal axis. Int Rev Cytol 213:183–232
Byrne M, Emlet RB, Cerra A (2001) Ciliated band structure in planktotrophic and lecithotrophic larvae of Heliocidaris species (Echinodermata: echinoidea): a demonstration of conservation and change. Acta Zool 82:189–199
Cameron CB, Garey JR, Swalla BJ (2000) Evolution of the chordate body plan: new insights from phylogenetic analyses of deuterostome phyla. Proc Natl Acad Sci U S A 97:4469–4474
Chia FS, Walker CW (1991) Echinodermata: asteroidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 6. Echinoderms and lophophorates. The Boxwood Press, Pacific Grove, CA, pp 301–353
Davidson EH, Peterson KJ, Cameron RA (1995) Origin of bilaterian body plans: evolution of developmental regulatory mechanisms. Science 270:1319–1325
Emlet RB (1988) Larval form and metamorphosis of a “primitive” sea urchin, Eucidaris thouarsi (Echinodermata: Echinoidea: Cidaroida), with implications for developmental and phylogenetic studies. Biol Bull 174:4–19
Emlet RB (1995) Larval spicules, cilia, and symmetry as remnants of indirect development in the direct developing sea urchin Heliocidaris erythrogramma. Dev Biol 167:405–415
Ferkowicz MJ, Raff RA (2001) Wnt gene expression in sea urchin development: heterochronies associated with the evolution of developmental mode. Evol Dev 3:24–33
Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ (2004) Origins of bilateral symmetry: Hox and Dpp expression in a sea anemone. Science 304:1335–1337
Gerhart J (2000) Inversion of the chordate body axis: are there alternatives? Proc Natl Acad Sci U S A 97:4445–4448
Haag ES, Raff RA (1998) Isolation and characterization of three mRNAs enriched in embryos of the direct-developing sea urchin Heliocidaris erythrogramma: evolution of larval ectoderm. Dev Genes Evol 208:188–204
Hadfield MG (1975) Hemichordata. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates, vol. 2. Entoprocts and lesser coelomates. Academic, New York, pp 185–240
Hendler G (1991) Echinodermata: ophiuroidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol. 6. Echinoderms and lophophorates. The Boxwood Press, Pacific Grove, CA, pp 355–511
Henry JJ (1998) The development of dorsoventral and bilateral axial properties in sea urchin embryos. Semin Cell Dev Biol 9:43–52
Henry JJ, Raff RA (1990) Evolutionary change in the process of dorsoventral axis determination in the direct developing sea urchin, Heliocidaris erythrogramma. Dev Biol 141:55–69
Henry JJ, Raff RA (1994) Progressive determination of cell fates along the dorsoventral axis in the sea urchin Heliocidaris erythrogramma. Rouxs Arch Dev Biol 204:62–69
Henry JJ, Wray GA, Raff RA (1990) The dorsoventral axis is specified prior to first cleavage in the direct developing sea urchin Heliocidaris erythrogramma. Development 110:875–884
Henry JJ, Wray GA, Raff RA (1991) Mechanism of an alternate type of echinoderm blastula formation: the wrinkled blastula of the sea urchin Heliocidaris erythrogramma. Dev Growth Differ 33:317–328
Holland ND (1991) Echinodermata: crinoidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 6. Echinoderms and lophophorates. The Boxwood Press, Pacific Grove, CA, pp 247–299
Hörstadius S (1973) Experimental embryology of echinoderms. Clarendon, Oxford
Hyman LH (1955) The invertebrates, vol. 4. Echinodermata. McGraw-Hill, New York
Hyman LH (1959) The invertebrates, vol. 5. Smaller coelomate groups. McGraw-Hill, New York
Janies DA, McEdward LR (1993) Highly derived coelomic and water-vascular morphogenesis in a starfish with pelagic direct development. Biol Bull 185:56–76
Kauffman JS, Raff RA (2003) Patterning mechanisms in the evolution of derived developmental life histories: the role of Wnt signaling in axis formation of the direct-developing sea urchin Heliocidaris erythrogramma. Dev Genes Evol 213:612–624
Kerr AM, Kim J (1999) Bi-penta-bi-decaradial symmetry: a review of evolutionary and developmental trends in Holothuroidea (Echinodermata). J Exp Zoolog Part B Mol Dev Evol 285:93–103
MacBride EW (1903) The development of Echinus esculentus, together with some points in the development of E. miliaris and E. acutus. Philos Trans R Soc Lond B Biol Sci 195:285–327
McCain ER, McClay DR (1994) The establishment of bilateral asymmetry in sea urchin embryos. Development 120:395–404
McEdward LR (1992) Morphology and development of a unique type of pelagic larva in the starfish Pteraster tesselatus (Echinodermata: Asteroidea). Biol Bull 182:177–187
Minsuk SB, Raff RA (2002) Pattern formation in a pentameral animal: induction of early adult rudiment development in sea urchins. Dev Biol 247:335–350
Minsuk SB, Raff RA (2005) Co-option of an oral-aboral patterning mechanism to control left–right differentiation: the direct-developing sea urchin Heliocidaris erythrogramma is sinistralized, not ventralized, by NiCl2. Evol Dev (in press)
Nakano H, Hibino T, Oji T, Hara Y, Amemiya S (2003) Larval stages of a living sea lily (stalked crinoid echinoderm). Nature 421:158–160
Okazaki K (1975) Normal development to metamorphosis. In: Czihak G (ed) The sea urchin embryo. Springer, Berlin Heidelberg New York, pp 177–232
Pearse JS, Cameron RA (1991) Echinodermata: echinoidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol. 6 Echinoderms and lophophorates. The Boxwood Press, Pacific Grove, CA, pp 513–662
Peterson KJ, Cameron RA, Davidson EH (1997) Set-aside cells in maximal indirect development: evolutionary and developmental significance. BioEssays 19:623–631
Peterson KJ, Cameron RA, Tagawa K, Satoh N, Davidson EH (1999a) A comparative molecular approach to mesodermal patterning in basal deuterostomes: the expression pattern of Brachyury in the enteropneust hemichordate Ptychodera flava. Development 126:85–95
Peterson KJ, Harada Y, Cameron RA, Davidson EH (1999b) Expression pattern of Brachyury and Not in the sea urchin: comparative implications for the origins of mesoderm in the basal deuterostomes. Dev Biol 207:419–431
Peterson KJ, Cameron RA, Davidson EH (2000) Bilaterian origins: significance of new experimental observations. Dev Biol 219:1–17
Popodi E, Raff RA (2001) Hox genes in a pentameral animal. BioEssays 23:211–214
Poznanski A, Minsuk S, Stathopoulos D, Keller R (1997) Epithelial cell wedging and neural trough formation are induced planarly in Xenopus, without persistent vertical interactions with mesoderm. Dev Biol 189:256–269
Raff RA (1996) The shape of life. The University of Chicago Press, Chicago
Sly BJ, Snoke MS, Raff RA (2003) Who came first—larvae or adults? Origins of bilaterian metazoan larvae. Int J Dev Biol 47:623–632
Smiley S (1986) Metamorphosis of Stichopus californicus (Echinodermata: Holothuroidea) and its phylogenetic implications. Biol Bull 171:611–631
Smiley S, McEuen FS, Chafee C, Krishnan S (1991) Echinodermata: holothuroidea. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 6. Echinoderms and lophophorates. The Boxwood Press, Pacific Grove, CA, pp 663–750
Summers RG, Piston DW, Harris KM, Morrill JB (1996) The orientation of first cleavage in the sea urchin embryo, Lytechinus variegatus, does not specify the axes of bilateral symmetry. Dev Biol 175:177–183
Urata M, Yamaguchi M (2004) The development of the enteropneust hemichordate Balanoglossus misakiensis Kuwano. Zool Sci 21:533–540
Williams DHC, Anderson DT (1975) The reproductive system, embryonic development, larval development and metamorphosis of the sea urchin Heliocidaris erythrogramma (Val.) (Echinoidea: Echinometridae). Aust J Zool 23:371–403
Wray GA (1996) Parallel evolution of nonfeeding larvae in echinoids. Syst Biol 45:308–322
Wray GA, Raff RA (1990) Novel origins of lineage founder cells in the direct-developing sea urchin Heliocidaris erythrogramma. Dev Biol 141:41–54
Wray GA, Raff RA (1991) Rapid evolution of gastrulation mechanisms in a sea urchin with lecithotrophic larvae. Evolution 45:1741–1750
Acknowledgements
We thank Ellen Larsen, Maria Byrne, and Ulrich Krohs for comments on the manuscript and helpful discussion. Thanks also to the Sydney Aquarium and the School of Biological Sciences, University of Sydney, for providing resources and for making our work in Australia possible. New South Wales Fisheries provided permits for collecting sea urchins. This work was funded by an NIH Postdoctoral Fellowship to S.B.M. and an NSF research grant to R.A.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Satoh
Rights and permissions
About this article
Cite this article
Minsuk, S.B., Andrews, M.E. & Raff, R.A. From larval bodies to adult body plans: patterning the development of the presumptive adult ectoderm in the sea urchin larva. Dev Genes Evol 215, 383–392 (2005). https://doi.org/10.1007/s00427-005-0486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-005-0486-9