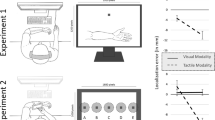
Abstract
The displacement of the final position of a moving object in the direction of the observed motion path, i.e. an overestimation, is known as representational momentum. It has been described both in the visual and the auditory domain, and is suggested to be modality-independent. Here, we tested whether a representational momentum can also be demonstrated in the somatosensory domain. While the cognitive literature on representational momentum suggests that it can, previous work on the psychophysics of tactile motion perception would rather predict an underestimation of the perceived endpoint of a tactile stimulus. Tactile motion stimuli were applied on the left and the right dorsal forearms of 32 healthy participants, who were asked to indicate the subjectively perceived endpoint of the stimulation. Velocity, length and direction of the trajectory were varied. Contrary to the prediction based on the representational momentum literature, participants in our experiment significantly displaced the endpoint against the direction of movement (underestimation). The results are thus compatible with previous psychophysical findings on the perception of tactile motion. Further studies combining paradigms from classical psychophysics and cognitive psychology will be needed to resolve the apparently paradoxical predictions by the two literatures.


Similar content being viewed by others
References
Actis-Grosso, R., & Stucchi, N. (2003). Shifting the start: Backward mislocation of the initial position of a motion. Journal of Experimental Psychology Human Perception and Performance, 29(3), 675–691.
Bolognini, N., Casanova, D., Maravita, A., & Vallar, G. (2012). Bisecting real and fake body parts: Effects of prism adaptation after right brain damage. Frontiers in Human Neuroscience, 6, 154. doi:10.3389/fnhum.2012.00154.
Brehaut, J. C., & Tipper, S. P. (1996). Representational momentum and memory for luminance. Journal of Experimental Psychology Human Perception and Performance, 22(2), 480–501.
Brouwer, A.-M., Franz, V. H., & Thornton, I. M. (2004). Representational momentum in perception and grasping: Translating versus transforming objects. Journal of Vision, 4(7), 575–584. doi:10.1167/4.7.5.
Brugger, P., & Meier, R. (2015). A new illusion at your elbow. Perception, 44(2), 219–221. doi:10.1068/p7910.
Cai, R. H., Jacobson, K., Baloh, R., Schlag-Rey, M., & Schlag, J. (2000). Vestibular signals can distort the perceived spatial relationship of retinal stimuli. Experimental Brain Research, 135(2), 275–278. doi:10.1007/s002210000549.
Cavanagh, P., & Anstis, S. (2013). The flash grab effect. Vision Research, 91, 8–20. doi:10.1016/j.visres.2013.07.007.
Cellini, C., Scocchia, L., & Drewing, K. (2016). The buzz-lag effect. Experimental Brain Research, 234(10), 2849–2857. doi:10.1007/s00221-016-4687-4.
Chapman, L. J., & Chapman, J. P. (1987). The measurement of handedness. Brain and Cognition, 6(2), 175–183.
Christopher Bill, J., & Teft, L. W. (1972). Space–time relations: The effects of variations in stimulus and interstimulus interval duration on perceived visual extent. Acta Psychologica, 36(5), 358–369. doi:10.1016/0001-6918(72)90032-7.
Cody, F. W. J., Garside, R. A. D., Lloyd, D., & Poliakoff, E. (2008). Tactile spatial acuity varies with site and axis in the human upper limb. Neuroscience Letters, 433(2), 103–108. doi:10.1016/j.neulet.2007.12.054.
Essick, G. K. (1998). Factors affecting direction discrimination of moving tactile stimuli. ADVANCES IN PSYCHOLOGY-AMSTERDAM-, 127, 1–54.
Essick, G. K., Bredehoeft, K. R., McLaughlin, D. F., & Szaniszlo, J. A. (1991). Directional sensitivity along the upper limb in humans. Somatosensory and Motor Research, 8(1), 13–22.
Essick, G. K., McGlone, F., Dancer, C., Fabricant, D., Ragin, Y., Phillips, N., & Guest, S. (2010). Quantitative assessment of pleasant touch. Neuroscience and Biobehavioral Reviews, 34(2), 192–203. doi:10.1016/j.neubiorev.2009.02.003.
Freyd, J. J. (1992). Dynamic representations guiding adaptive behavior. Time, action and cognition (pp. 309–323). Berlin: Springer.
Freyd, J. J., & Finke, R. A. (1984). Facilitation of length discrimination using real and imaged context frames. The American Journal of Psychology, 97(3), 323–341.
Freyd, J. J., & Johnson, J. Q. (1987). Probing the time course of representational momentum. Journal of Experimental Psychology Learning Memory and Cognition, 13(2), 259–268. doi:10.1037/0278-7393.13.2.259.
Freyd, J. J., Kelly, M. H., & DeKay, M. L. (1990). Representational momentum in memory for pitch. Journal of Experimental Psychology Learning Memory and Cognition, 16(6), 1107–1117. doi:10.1037/0278-7393.16.6.1107.
Gallace, A., & Spence, C. (2010). The science of interpersonal touch: An overview. Neuroscience and Biobehavioral Reviews, 34(2), 246–259. doi:10.1016/j.neubiorev.2008.10.004.
Getzmann, S., & Lewald, J. (2009). Constancy of target velocity as a critical factor in the emergence of auditory and visual representational momentum. Experimental Brain Research, 193(3), 437–443. doi:10.1007/s00221-008-1641-0.
Getzmann, S., Lewald, J., & Guski, R. (2004). Representational momentum in spatial hearing. Perception, 33(5), 591–599.
Goldreich, D. (2007). A Bayesian perceptual model replicates the cutaneous rabbit and other tactile spatiotemporal illusions. PLoS One, 2(3), e333. doi:10.1371/journal.pone.0000333.
Goldreich, D., & Tong, J. (2013). Prediction, postdiction, and perceptual length contraction: A Bayesian low-speed prior captures the cutaneous rabbit and related illusions. Consciousness Research, 4, 221. doi:10.3389/fpsyg.2013.00221.
Hall, G. S., & Donaldson, H. H. (1885). Motor Sensations on the Skin. Mind, 10(40), 557–572.
Helson, H. (1930). The Tau effect—an example of psychological relativity. Science, 71(1847), 536–537. doi:10.1126/science.71.1847.536.
Hohwy, J. (2013). The predictive mind. Oxford: Oxford University Press.
Hubbard, T. L. (1990). Cognitive representation of linear motion: Possible direction and gravity effects in judged displacement. Memory and Cognition, 18(3), 299–309.
Hubbard, T. L. (1995a). Auditory representational momentum: Surface form, direction, and velocity effects. The American Journal of Psychology, 108(2), 255–274. doi:10.2307/1423131.
Hubbard, T. L. (1995b). Cognitive representation of motion: Evidence for friction and gravity analogues. Journal of Experimental Psychology Learning Memory and Cognition, 21(1), 241.
Hubbard, T. L. (1995c). Environmental invariants in the representation of motion: Implied dynamics and representational momentum, gravity, friction, and centripetal force. Psychonomic Bulletin and Review, 2(3), 322–338.
Hubbard, T. L. (2005). Representational momentum and related displacements in spatial memory: A review of the findings. Psychonomic Bulletin and Review, 12(5), 822–851. doi:10.3758/BF03196775.
Hubbard, T. L. (2014). Forms of momentum across space: Representational, operational, and attentional. Psychonomic Bulletin and Review, 21(6), 1371–1403. doi:10.3758/s13423-014-0624-3.
Hubbard, T. L., & Bharucha, J. J. (1988). Judged displacement in apparent vertical and horizontal motion. Perception and Psychophysics, 44(3), 211–221.
Hubbard, T. L., & Motes, M. A. (2002). Does representational momentum reflect a distortion of the length or the endpoint of a trajectory? Cognition, 82(3), 89–99.
Hubbard, T. L., & Motes, M. A. (2005). An effect of context on whether memory for initial position exhibits a Fröhlich effect or an onset repulsion effect. The Quarterly Journal of Experimental Psychology A Human Experimental Psychology, 58(6), 961–979. doi:10.1080/02724980443000368.
Intraub, H. (2004). Anticipatory spatial representation of 3D regions explored by sighted observers and a deaf-and-blind-observer. Cognition, 94(1), 19–37.
Intraub, H., Morelli, F., & Gagnier, K. M. (2015). Visual, haptic and bimodal scene perception: Evidence for a unitary representation. Cognition, 138, 132–147. doi:10.1016/j.cognition.2015.01.010.
Johnston, H. M., & Jones, M. R. (2006). Higher order pattern structure influences auditory representational momentum. Journal of Experimental Psychology Human Perception and Performance, 32(1), 2–17. doi:10.1037/0096-1523.32.1.2.
Kennett, S., Taylor-Clarke, M., & Haggard, P. (2001). Noninformative vision improves the spatial resolution of touch in humans. Current Biology, 11(15), 1188–1191.
Kerzel, D. (2000). Eye movements and visible persistence explain the mislocalization of the final position of a moving target. Vision Research, 40(27), 3703–3715. doi:10.1016/S0042-6989(00)00226-1.
Kerzel, D. (2002). A matter of design: No representational momentum without predictability. Visual Cognition, 9(1–2), 66–80.
Kerzel, D. (2003). Mental extrapolation of target position is strongest with weak motion signals and motor responses. Vision Research, 43(25), 2623–2635.
Kerzel, D., Jordan, S., & Müsseler, J. (2001). The role of perception in the mislocalization of the final position of a moving target. Journal of Experimental Psychology Human Perception and Performance, 27(4), 829–840. doi:10.1037/0096-1523.27.4.829.
Langford, N., Hall, R. J., & Monty, R. A. (1973). Cutaneous perception of a track produced by a moving point across the skin. Journal of Experimental Psychology, 97(1), 59. doi:10.1037/h0033767.
Lawrence, M. A. (2016). ez: Easy analysis and visualization of factorial experiments (Version 4.4-0). https://cran.r-project.org/web/packages/ez/index.html. Accessed 31 Jan 2017.
Lenggenhager, B., Loetscher, T., Kavan, N., Pallich, G., Brodtmann, A., Nicholls, M. E. R., & Brugger, P. (2012). Paradoxical extension into the contralesional hemispace in spatial neglect. Cortex A Journal Devoted to the Study of the Nervous System and Behavior, 48(10), 1320–1328. doi:10.1016/j.cortex.2011.10.003.
Nijhawan, R. (2002). Neural delays, visual motion and the flash-lag effect. Trends in Cognitive Sciences, 6(9), 387.
Nijhawan, R., & Kirschfeld, K. (2003). Analogous mechanisms compensate for neural delays in the sensory and the motor pathways. Current Biology, 13(9), 749–753. doi:10.1016/S0960-9822(03)00248-3.
Pack, C. C., & Bensmaia, S. J. (2015). Seeing and feeling motion: Canonical computations in vision and touch. PLoS Biology, 13(9), e1002271. doi:10.1371/journal.pbio.1002271.
Phillips, N. (2016). yarrr: A companion to the e-book YaRrr!: The Pirate’s Guide to R. https://cran.r-project.org/web/packages/yarrr/index.html. Accessed 2 May 2016.
R Core Team. (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria. http://www.r-project.org/. Accessed 4 Nov 2015.
Sarrazin, J.-C., Giraudo, M.-D., & Pittenger, J. B. (2005). Tau and Kappa effects in physical space: The case of audition. Psychological Research, 71(2), 201–218. doi:10.1007/s00426-005-0019-1.
Schlag, J., Cai, R. H., Dorfman, A., Mohempour, A., & Schlag-Rey, M. (2000). Extrapolating movement without retinal motion. Nature, 403(6765), 38–39. doi:10.1038/47402.
Seizova-Cajic, T., & Taylor, J. L. (2014). Somatosensory space abridged: Rapid change in tactile localization using a motion stimulus. PLoS One, 9(3), e90892. doi:10.1371/journal.pone.0090892.
Senna, I., Parise, C. V., & Ernst, M. O. (2015). Hearing in slow-motion: Humans underestimate the speed of moving sounds. Scientific Reports, 5, 14054. doi:10.1038/srep14054.
Tapley, S. M., & Bryden, M. P. (1985). A group test for the assessment of performance between the hands. Neuropsychologia, 23(2), 215–221.
Thornton, I. M. (2014). Representational momentum and the human face: An empirical note. WICT, 2014, 101.
Tong, J., Ngo, V., & Goldreich, D. (2016). Tactile length contraction as Bayesian inference. Journal of Neurophysiology. doi:10.1152/jn.00029.2016.
Trojan, J., Kleinböhl, D., Stolle, A. M., Andersen, O. K., Hölzl, R., & Arendt-Nielsen, L. (2006). Psychophysical “perceptual maps” of heat and pain sensations by direct localization of CO2 laser stimuli on the skin. Brain Research, 1120(1), 106–113. doi:10.1016/j.brainres.2006.08.065.
Whitsel, B. L., Favorov, O. V., Kelly, D. G., & Tommerdahl, M. (1991). Mechanisms of dynamic peri- and intra-columnar interactions in somatosensory cortex: Stimulus-specific contrast enhancement by NMDA receptor activation. In O. Frazen & J. Westman (Eds.), Information processing in the somatosensory system (pp. 353–369). London: Macmillan Press.
Whitsel, B. L., Franzen, O., Dreyer, D. A., Hollins, M., Young, M., Essick, G. K., & Wong, C. (1986). Dependence of subjective traverse length on velocity of moving tactile stimuli. Somatosensory Research, 3(3), 185–196.
Wickham, H. (2016). tidyverse: Easily install and load “Tidyverse” packages (Version 1.1.1). https://cran.r-project.org/web/packages/tidyverse/index.html. Accessed 31 Jan 2017.
Yoshikawa, S., & Sato, W. (2008). Dynamic facial expressions of emotion induce representational momentum. Cognitive Affective and Behavioral Neuroscience, 8(1), 25–31.
Acknowledgements
We thank Stefan Engelter, senior neurologist at University Hospital Basel, for encouraging us to publish this experiment. We thank Peter Rohner for the illustration of the experimental setup and Daniel Goldreich for his comments on a previous version of the manuscript. Finally, the very constructive critique of three expert reviewers is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local Ethics Committee of the University of Basel. All participants gave written informed consent before the experiment.
Rights and permissions
About this article
Cite this article
Macauda, G., Lenggenhager, B., Meier, R. et al. Tactile motion lacks momentum. Psychological Research 82, 889–895 (2018). https://doi.org/10.1007/s00426-017-0879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-017-0879-1