Abstract
Main conclusion
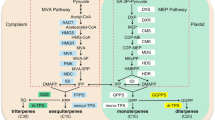
A novel terpene synthase ( Tps ) gene isolated from Camellia brevistyla was identified as hedycaryol synthase, which was shown to be expressed specifically in flowers.
Camellia plants are very popular because they bloom in winter when other plants seldom flower. Many ornamental cultivars of Camellia have been bred mainly in Japan, although the fragrance of their flowers has not been studied extensively. We analyzed floral scents of several Camellia cultivars by gas chromatography–mass spectrometry (GC–MS) and found that Camellia brevistyla produced various sesquiterpenes in addition to monoterpenes, whereas Camellia japonica and its cross-lines produced only monoterpenes, including linalool as the main product. From a flower of C. brevistyla, we isolated one cDNA encoding a terpene synthase (TPS) comprised of 554 amino acids, which was phylogenetically positioned to a sole gene clade. The cDNA, designated CbTps1, was expressed in mevalonate-pathway-engineered Escherichia coli, which carried the Streptomyces mevalonate-pathway gene cluster in addition to the acetoacetate-CoA ligase gene. A terpene product was purified from recombinant E. coli cultured with lithium acetoacetate, and analyzed by 1H-nulcear magnetic resonance spectroscopy (1H-NMR) and GC–MS. It was shown that a sesquiterpene hedycaryol was produced, because 1H-NMR signals of the purified product were very broad, and elemol, a thermal rearrangement product from hedycaryol, was identified by GC–MS analysis. Spectroscopic data of elemol were also determined. These results indicated that the CbTps1 gene encodes hedycaryol synthase. Expression analysis of CbTps1 showed that it was expressed specifically in flowers, and hedycaryol is likely to be one of the terpenes that attract insects for pollination of C. brevistyla. A linalool synthase gene, which was isolated from a flower of Camellia saluenensis, is also described.







Similar content being viewed by others
Abbreviations
- Cm:
-
Chloramphenicol
- DMAPP:
-
Dimethylallyl diphosphate
- FPP:
-
Farnesyl diphosphate
- GC:
-
Gas chromatography
- GC-MS:
-
Gas chromatography–mass spectrometry
- GPP:
-
Geranyl diphosphate
- HPLC:
-
High-performance liquid chromatography
- IPP:
-
Isopentenyl diphosphate
- IPTG:
-
Isopropyl β-d-thiogalactopyranoside
- Km:
-
Kanamycin
- LAA:
-
Lithium acetoacetate
- MEP:
-
Methylerythritol phosphate
- MVA:
-
Mevalonate
- NMR:
-
Nuclear magnetic resonance spectroscopy
- ORF:
-
Open-reading frame
- RACE:
-
Rapid amplification of cDNA ends
- RT:
-
Reverse transcription
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- SPME:
-
Solid phase micro-extraction
- TPS:
-
Terpene synthase
References
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95:4126–4133
Chandler SF, Sanchez C (2012) Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotechnol J 10:891–903
Chen L, Zhou Z-X, Yang Y-J (2006) Genetic improvement and breeding of tea plant (Camellia sinensis) in China: from individual selection to hybridization and molecular breeding. Int J Plant Breed. doi:10.1007/s10681-006-9292-3/fulltext.html
Dobson HEM (2006) Relationship between floral fragrance composition and type of pollinator. In: Dudareva N, Pichersky E (eds) Biology of floral scent. CRC Press, New York, pp 147–196
Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Fäldt J, Miller B, Bohlmann J (2003) (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15:1227–1241
Faraldos JA, Wu S, Chappell J, Coates RM (2007) Conformational analysis of (+)-germacrene A by variable-temperature NMR and NOE spectroscopy. Tetrahedron 63:7733–7742
Fujisawa M, Harada H, Kenmoku H, Mizutani S, Misawa N (2010) Cloning and characterization of a novel gene that encodes (S)-β-bisabolene synthase from ginger, Zingiber officinale. Planta 232:121–131
Gennadios HA, Gonzalez V, Costanzo LD, Li A, Yu F, Miller DJ, Allemann RK, Christianson DW (2009) Crystal structure of (+)-δ-cadinene synthase from Gossypium arboreum and evolutionary divergence of metal binding motifs for catalysis. Biochemistry 48:6175–6183
George KW, Alonso-Gutierrez J, Keasling JD, Lee TS (2015) Isoprenoid drugs, biofuels, and chemicals—artemisinin, farnesene, and beyond. Adv Biochem Eng Biotechnol. doi:10.1007/10_2014_288
Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A (2008) Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA 105:9099–9104
Ginglinger JF, Boachon B, Höfer R, Paetz C, Köllner TG, Miesch L, Lugan R, Baltenweck R, Mutterer J, Ullmann P, Beran F, Claudel P, Verstappen F, Fischer MJC, Karst F, Bouwmeester H, Miesch M, Schneider B, Gershenzon J, Ehlting J, Werck-Reichhart D (2013) Gene coexpression analysis reveals complex metabolism of the monoterpene alcohol linalool in Arabidopsis flowers. Plant Cell 25:4640–4657
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Harada H, Misawa N (2009) Novel approaches and achievements in biosynthesis of functional isoprenoids in Escherichia coli. Appl Microbiol Biotechnol 84:1021–1031
Harada H, Yu F, Okamoto S, Kuzuyama T, Utsumi R, Misawa N (2009) Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl Microbiol Biotechnol 81:915–925
Hartmann T (1996) Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol Ex Appl 80:177–188
Hattan J, Misawa N (2015) Production of functional isoprenoids through pathway engineering. In: Grunwald P (ed) Industrial biocatalysis, Pan Stanford Series on biocatalysis, vol 1. Pan Stanford Publishing Pte Ltd, Stanford, pp 161–179
Irmisch S, Jiang Y, Chen F, Gershenzon J, Köllner TG (2014) Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa). BMC Plant Biol 14:270
Jackson AJ, Hershey DM, Chesnut T, Xu M, Peters RJ (2014) Biochemical characterization of the castor bean ent-kaurene synthase(-like) family supports quantum chemical view of diterpene cyclization. Phytochemistry 103:13–21
Japan Camellia Soc (2010) Camellias of Japan. Seibundo-shinkosha, Tokyo (ISBN978-4-416-41006-6)
Jones RVH, Sutherland MD (1968) Hedycaryol, the precursor of elemol. Chem Commun (Lond) 20:1229–1230
Jones CG, Moniodis J, Zulak KG, Scaffidi A, Plummer JA, Ghisalberti EL, Barbour EL, Bohlmann J (2011) Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b subfamilies, including santalene synthases. J Biol Chem 286:17445–17454
Jullien F, Gao J, Orel G, Legendre L (2008) Analysis of tissue-specific emission of volatiles by the flowers of six Camellia species. Flavour Fragr J 23:115–120
Kirby J, Keasling JD (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60:335–355
Knudsen JT, Gershenzon J (2006) The chemical diversity of floral scent. In: Dudareva N, Pichersky E (eds) Biology of floral scent. CRC Press, New York, pp 27–52
Knudsen JT, Eriksson R, Gershenzon J, Ståhl B (2006) Diversity and distribution of floral scent. Bot Rev 72:1–120
Köksal M, Hu H, Coates RM, Peters RJ, Christianson DW (2011) Structure and mechanism of the diterpene cyclase ent-copalyl diphosphate synthase. Nat Chem Biol 7:431–433
Koo HJ, Gang DR (2012) Suites of terpene synthases explain differential terpenoid production in ginger and turmeric tissues. PLoS One 7:e51481
Li JB, Hashimoto F, Shimizu K, Sakata Y (2013) Chemical taxonomy of red-flowered wild Camellia species based on floral anthocyanins. Phytochemistry 85:99–106
Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796–802
Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, Toub O, Lund ST, Bohlmann J (2010) Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol 10:226
Matsunaga T, Hasegawa C, Kawasuji T, Sizuki H, Saito H, Sagioka T, Takahashi R, Tsukamoto H, Morikawa T, Akiyama T (2000) Isolation of the antiulcer compound in essential oil from the leaves of Cryptomeria japonica. Biol Pharm Bull 23:595–598
Melillo E, Setroikromo R, Quax WJ, Kayser O (2013) Production of α-cuprenene in Xanthophyllomyces dendrorhous: a step closer to a potent terpene. Microb Cell Fact. doi:10.1186/1475-2859-12-13
Misawa N (2011) Pathway engineering for functional isoprenoids. Curr Opin Biotechnol 22:627–633
Miyazawa M, Watanabe H, Umemoto K, Kameoka H (1998) Inhibition of acetylcholinesterase activity by essential oils of Mentha species. J Agric Food Chem 46:3431–3434
Omata A, Yomogida K, Nakamura S, Ota T, Izawa Y (1989) Studies on the volatile compounds of Camellia flowers. J Jpn Soc Hortic Sci 58:429–434
Osawa E, Shimada K, Kodama M, Ito S (1979) Conformation of hedycaryol isomers. Tetrahedron Lett 20:2353–2354
Oyama-Okubo N, Tanikawa N, Nakayama M, Shibata M, Suzuki K, Kondo M (2009) Screening of genetic resources of Camellia lutchuensis for fragrant Camellia breeding; analysis of floral scent compounds. Proc Int Symp New Floricult Crops Acta Hortic 813:399–406
Rohloff J, Bones AM (2005) Volatile profiling of Arabidopsis thaliana—putative olfactory compounds in plant communication. Phytochemistry 66:1941–1955
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Saxena G, Banerjee S, Rahman L, Verma PC, Mallavarapu GR, Kumar S (2007) Rose-scented geranium (Pelargonium sp.) generated by Agrobacterium rhizogenes mediated Ri-insertion for improved essential oil quality. Plant Cell Tissue Organ Cult 90:215–223
Shibata M, Aida R, Kishimoto S, Tanikawa N, Onozaki T, Kayumi S (2004) Breeding process and characteristics of Camellia Norin No.4 ‘Himenoka’ by interspecific hybridization between Camellia japonica and C. lutchuensis. Bull Natl Inst Flor Sci 4:1–11
Tamaru S, Ohmachi K, Miyata Y, Tanaka T, Kubayasi T, Nagata Y, Tanaka K (2013) Hypotriglyceridemic potential of fermented mixed tea made with third-crop green tea leaves and Camellia (Camellia japonica) leaves in Sprague-Dawley rats. J Agric Food Chem 61:5817–5823
Tanikawa N, Ban Y, Morita Y, Nakayama M, Shibata M (2013) Identification of maternal ancestor species of Camellia cultivars ‘Robiraki’ and ‘Tagoto-no-tsuki’ by chloroplast DNA polymorphisms. Hortic Res (Jpn) 12:9–14
Thulasiram HV, Erickson HK, Poulter CD (2007) Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science 316:73–76
Tsuneki H, Ma EL, Kobayashi S, Sekizaki N, Maekawa K, Sasaoka T, Wang MW, Kimura I (2005) Antiangiogenic activity of β-eudesmol in vitro and in vivo. Eur J Pharmacol 512:105–115
Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J (2006) Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotechnol 24:1441–1447
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ, Xu YH (2008) Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett 264:127–134
Yoshikuni Y, Ferrin TE, Keasling JD (2006) Designed divergent evolution of enzyme function. Nature 440:1078–1082
Yu F, Harada H, Yamasaki K, Okamoto S, Hirase S, Tanaka Y, Misawa N, Utsumi R (2008a) Isolation and functional characterization of a beta-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Lett 582:565–572
Yu F, Okamoto S, Nakasone K, Adachi K, Matsuda S, Harada H, Misawa N, Utsumi R (2008b) Molecular cloning and functional characterization of α-humulene synthase, a possible key enzyme of zerumbone biosynthesis in shampoo ginger (Zingiber zerumbet Smith). Planta 227:1291–1299
Acknowledgments
We thank Mr. Seizou Matsui, Kanazawa branch of the Japan Camellia Association, for kindly providing the C. brevistyla and other Camellia samples and important information on Camellia plants. We also thank to Dr. Eiji Tanaka, Ishikawa Prefectural University, for advice on manuscript preparation. We are grateful to Takasago International Corporation (Tokyo, Japan) for kindly providing the authentic preparation of elemol. This work was supported by the commission for Development of Artificial Gene Synthesis Technology for Creating Innovative Biomaterial from the Ministry of Economy, Trade and Industry (METI), Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hattan, Ji., Shindo, K., Ito, T. et al. Identification of a novel hedycaryol synthase gene isolated from Camellia brevistyla flowers and floral scent of Camellia cultivars. Planta 243, 959–972 (2016). https://doi.org/10.1007/s00425-015-2454-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2454-6