Abstract
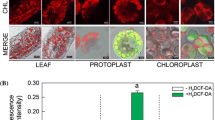
Despite extensive research over the past years, regeneration from protoplasts has been observed in only a limited number of plant species. Protoplasts undergo complex metabolic modification during their isolation. The isolation of protoplasts induces reactive oxygen species (ROS) generation in Brassica napus leaf protoplasts. The present study was conducted to provide new insight into the mechanism of ROS generation in B. napus leaf protoplasts. In vivo localization of H2O2 and enzymes involved in H2O2 generation and detoxification, molecular antioxidant-ascorbate and its redox state and lipid peroxidation were investigated in the leaf and isolated protoplasts. Incubating leaf strips in the macerating enzyme (ME) for different duration (3, 6, and 12 h) induced accumulation of H2O2 and malondialdehyde (lipid peroxidation, an index of membrane damage) in protoplasts. The level of H2O2 was highest just after protoplast isolation and subsequently decreased during culture. Superoxide generating NADPH oxidase (NOX)-like activity was enhanced, whereas superoxide dismutase (SOD) and ascorbate peroxidase (APX) decreased in the protoplasts compared to leaves. Diaminobenzidine peroxidase (DAB-POD) activity was also lower in the protoplasts compared to leaves. Total ascorbate content, ascorbate to dehydroascorbate ratio (redox state), were enhanced in the protoplasts compared to leaves. Higher activity of NOX-like enzyme and weakening in the activity of antioxidant enzymes (SOD, APX, and DAB-POD) in protoplasts resulted in excessive accumulation of H2O2 in chloroplasts of protoplasts. Chloroplastic NADPH oxidase-like activity mediated perpetual H2O2 generation probably induced apoptotic-like cell death of B. napus leaf protoplasts as indicated by parallel DNA laddering and decreased mitochondrial membrane potential.







Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- DAB-POD:
-
3,3-Diaminobenzidine peroxidase
- DHA:
-
Dehydroascorbic acid
- DiOC6 :
-
3,3′-Dihexyloxacarbocyanine iodide
- DPI:
-
Diphenyleneiodonium chloride
- G-POD:
-
Guaiacol peroxidase
- H2DCF-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- ME:
-
Macerating enzyme
- NOX:
-
NADPH oxidase
- PCD:
-
Programmed cell death
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- XTT:
-
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide sodium salt
References
Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9:1559–1572
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569:1–9
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and assays applicable to acrylamide gels. Anal Biochem 44:276–287
Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol 4:561–567
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bienert GP, Moller ALB, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaki M, Fernández-Ocaña AM, Valderrama R, Carreras A, Esteban FJ, Luque F, Gómez-Rodríguez MV, Begara-Morales JC, Corpas FJ, Barroso JB (2009) Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower-mildew interaction. Plant Cell Physiol 50:265–279
Cheeseman JM (2007) Hydrogen peroxide and plant stress: a challenging relationship. Plant Stress 1:4–15
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98:13454–13459
Doyle SM, Diamond M, McCabe PF (2010) Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J Exp Bot 61:473–482
Drew MC, He C-J, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5:123–127
Gao CJ, Xing D, Li LL, Zhang LR (2008) Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 227:755–767
Greenberg JT (1996) Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA 93:12094–12097
Halliwell B (2001) Vitamin C and genomic stability. Mutation Res 475:29–35
Halliwell B, Gutteridge J (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Hames BD (1990) One dimensional polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of protein. Oxford University Press, UK, pp 1–87
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:180–198
Ishii S (1987) Generation of active oxygen species during enzymic isolation of protoplasts from oat leaves. In Vitro Cell Dev Biol 23:653–658
Kieselbach T, Hagman Å, Andersson B, Schröder WP (1998) The thylakoid lumen of chloroplasts: isolation and characterization. J Biol Chem 273:6710–6716
Korn A, Ajlani G, Lagoutte B, Gall A, Sétif P (2009) Ferredoxin:NADP+ oxidoreductase association with phycocyanin modulates its properties. J Biol Chem 284:31789–31797
Kristiansen KA, Jensen PE, Møller IM, Schulz A (2009) Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant 136:369–383
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848–853
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts, the effect of hydrogen peroxide and of paraquat. Biochem J 210:899–903
Mittler R, Vanderauwera S, Gollery M, van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Papadakis AK, Siminis CI, Roubelakis-Angelakis KA (2001) Reduced activity of antioxidant machinery is correlated with suppression of totipotency in plant protoplasts. Plant Physiol 126:434–444
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J (1998) Vitamin C exhibits pro-oxidant properties. Nature 392:559
Rogers HJ (2006) Programmed cell death in floral organs: How and why do flowers die? Ann Bot 97:309–315
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Schimmel SD, Kent C, Bischoff R, Vagelos PR (1973) Plasma membranes from cultured muscle cells: isolation procedure and separation of putative plasma-membrane marker enzymes. Proc Natl Acad Sci USA 70:3195–3199
Simon-Plas F, Elmayan T, Blein JP (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31:137–147
Tewari RK, Kim S, Hahn EJ, Paek KY (2008) Involvement of nitric oxide-induced NADPH oxidase in adventitious root growth and antioxidant defence in Panax ginseng. Plant Biotechnol Rep 2:113–122
Tewari RK, Kumar P, Kim S, Hahn EJ, Paek KY (2009) Nitric oxide retards xanthine oxidase-mediated superoxide anion generation in Phalaenopsis flower: an implication of NO in the senescence and oxidative stress regulation. Plant Cell Rep 28:267–279
Thomas JC, Ughy B, Lagoutte B, Ajlani G (2006) A second isoform of the ferredoxin:NADP+ oxidoreductase generated by an in-frame initiation of translation. Proc Natl Acad Sci USA 103:18368–18373
Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99:517–522
Truitt CL, Wei HX, Pare PW (2004) A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 16:523–532
Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E (2006) Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock induced cell death. Plant Physiol 14:208–219
Vasil IK (1987) Developing cell and tissue culture systems for the improvement of cereal and grass crops. J Plant Physiol 128:193–218
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1468–1476
Watanabe M, Watanabe Y, Shimada N (1992) Colorimetric estimation of leaf-protoplast potential-to-divide by use of 2, 3, 5-triphenyl tetrazolium chloride. J Plant Physiol 44:111–114
Watanabe M, Kawasaki H, Itho Y, Watanabe Y (1998) Senescence development of Brassica napus leaf protoplasts during isolation and subsequent culture. J Plant Physiol 152:487–493
Watanabe M, Setoguchi D, Uehara K, Ohtsuka W, Watanabe Y (2002a) Apoptotic-like cell death of Brassica napus leaf protoplasts. New Phytol 156:417–426
Watanabe M, Suzuki K, Kawasaki H, Watanabe Y (2002b) Differential responses of Brassica napus and Petunia hybrida to leaf protoplast isolation stress. Physiol Plant 114:645–651
Yasuda K, Watanabe Y, Watanabe M (2007) Generation of intracellular reactive oxygen species during the isolation of Brassica napus leaf protoplasts. Plant Biotechnol 24:361–366
Yokota A, Kawabata A, Kitaoka S (1983) Mechanism of glyoxylate decarboxylation in the glycolate pathway in Euglena gracilis Z: participation of Mn2+-dependent NADPH oxidase in chloroplasts. Plant Physiol 71:772–776
Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15:706–718
Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inze′ D, Delledonne M, Van Breusegem F (2006) Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol 141:404–411
Zhang LR, Xing D (2008) Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49:1092–1111
Zhang H, Fang Q, Zhang Z, Wang Y, Zheng X (2009a) The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. J Exp Bot 60:3109–3122
Zhang L, Li Y, Xing D, Gao C (2009b) Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J Exp Bot 60:2073–2091
Acknowledgments
This work was financially supported by Japan Society for the Promotion of Science (JSPS) as a postdoctoral fellowship (P08413) to RKT and grant-in-aid for scientific research. Authors are thankful to Prof. Esso Nishino, Chiba University, for his help in preparation of microtome sections of leaf.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tewari, R.K., Watanabe, D. & Watanabe, M. Chloroplastic NADPH oxidase-like activity-mediated perpetual hydrogen peroxide generation in the chloroplast induces apoptotic-like death of Brassica napus leaf protoplasts. Planta 235, 99–110 (2012). https://doi.org/10.1007/s00425-011-1495-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1495-8