Abstract
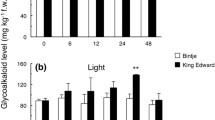
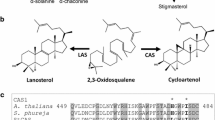
The potato steroidal glycoalkaloids (SGA) are toxic secondary metabolites, and their total content in tubers should not exceed 20 mg/100 g fresh weight. The two major SGA in cultivated potato (Solanum tuberosum) are α-chaconine and α-solanine. SGA biosynthetic genes and the genetic factors that control their expression have not yet been determined. In the present study, potato genotypes exhibiting different levels of SGA content showed an association between high SGA levels in their leaves and tubers and high expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 (hmg1) and squalene synthase 1 (pss1), genes of the mevalonic/isoprenoid pathway. Transcripts of other key enzymes of branches of the isoprenoid pathway, vetispiradiene/sesquiterpene synthase (pvs1) and sterol C24-methyltransferase type1 (smt1), were undetectable or exhibited stable expression regardless of SGA content, respectively, suggesting facilitated precursor flow to the SGA biosynthetic branch. The transcript ratio of solanidine glucosyltransferase (sgt2) to solanidine galactosyltransferase (sgt1) was correlated to the documented chaconine-to-solanine ratio in the tested genotypes. Significantly higher expression of hmg1, pss1, smt1, sgt1 and sgt2 was monitored in the tuber phelloderm than in the parenchyma of the tuber’s flesh, targeting the former as the main SGA-producing tissue in the tuber, in agreement with the known high SGA content in the layers directly under the tuber skin.






Similar content being viewed by others
Abbreviations
- SGA:
-
Steroidal glycoalkaloids
- HMGR:
-
3-Hydroxy-3-methylglutaryl coenzyme A reductase
- PSS:
-
Squalene synthase
- SMT1:
-
Sterol C24-methyltransferase type1
- SGT1:
-
Solanidine galactosyltransferase
- SGT2:
-
Solanidine glucosyltransferase
References
Arnqvist L, Dutta PC, Jonsson L, Sitbon F (2003) Reduction of cholesterol and glycoalkaloid levels in transgenic potato plants by overexpression of a type 1 sterol methyltransferase cDNA. Plant Physiol 131:1792–1799
Austin S, Lojkowska E, Ehlenfeldt MK, Kelman A, Helgeson JP (1988) Fertile interspecific somatic hybrids of Solanum: a novel source of resistance to Erwinia soft rot. Phytopathology 78:1216–1220
Bejarano L, Mignolet E, Devaux A, Espinola N, Carrasco E, Larondelle Y (2000) Glycoalkaloids in potato tubers: the effect of variety and drought stress on the alpha-solanine and alpha-chaconine contents of potatoes. J Sci Food Agric 80:2096–2100
Bergenstråhle A, Tillberg E, Jonsson L (1992) Regulation of glycoalkaloid accumulation in potato tuber disks. J Plant Physiol 140:269–275
Bouarte-Medina T, Fogelman E, Chani E, Miller AR, Levin I, Levy D, Veilleux RE (2002) Identification of molecular markers associated with leptine in reciprocal backcross families of diploid potato. Theor Appl Genet 105:1010–1018
Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate limiting step for isoprenoid biosynthesis in plants? Plant Physiol 109:1337–1343
Choi D, Ward BL, Bostock RM (1992) Differential induction and suppression of potato 3-hydroxy-3-methylglutaryl coenzyme A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid. Plant Cell 4:1333–1344
Choi D, Bostock RM, Avdiushko S, Hildebrand DF (1994) Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants: methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc Nat Acad Sci USA 91:2329–2333
Dale MFB, Griffiths DW, Bain H, Todd D (1993) Glycoalkaloid increase in Solanum tuberosum on exposure to light. Ann Appl Biol 123:411–418
Dale S, Arro M, Becerra B, Morrice NG, Boronat A, Hardie DG, Ferrer A (1995) Bacterial expression of the catalytic domain of 3-hydroxy-3-methylglutaryl Co-A reductase (isoform HMGR1) from Arabidopsis thaliana, and its inactivation by phosphorylation at Ser577 by Brassica oleracea 3-hydroxy-3-methylglutaryl CoA reductase kinase. Eur J Biochem 233:506–513
Dimenstein L, Lisker N, Kedar N, Levy D (1997) Changes in the content of steroidal glycoalkaloids in potato tubers grown in the field and in the greenhouse under different conditions of light, temperature and daylength. Physiol Mol Plant Pathol 50:391–402
Fewell AM, Roddick JG (1997) Potato glycoalkaloid impairment of fungal development. Mycol Res 101:597–603
Friedman M, McDonald GM (1997) Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit Rev Plant Sci 16:55–132
Friedman M, Roitman JN, Kozukue N (2003) Glycoalkaloid and calystegine contents of eight potato cultivars. J Agric Food Chem 51:2964–2973
Friedman M, Lee KR, Kim HJ, Lee IS, Kozukue N (2005) Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells. J Agric Food Chem 53:6162–6169
Heftmann E (1983) Biogenesis of steroids in Solanaceae. Phytochemistry 22:1843–1860
Kondo K, Uritani I, Oba K (2003) Induction mechanism of 3-hydroxy-3-methylglutaryl-CoA reductase in potato tuber and sweet potato root tissues. Biosci Biotechnol Biochem 67:1007–1017
Korth KL, Jaggard DAW, Dixon RA (2000) Developmental and light-regulated post-translational control of 3-hydroxy-3-methylglutaryl-CoA reductase levels in potato. Plant J 23:507–516
Lafta AM, Lorenzen JH (2000) Influence of high temperature and reduced irradiance on glycoalkaloid levels in potato leaves. J Am Soc Hortic Sci 125:563–566
Lawson DR (1993) Chemistry and biochemistry of Solanum chacoense, bitter steroidal alkaloids. PhD thesis, Ohio State University
Lulai EC, Freeman TP (2001) The importance of phellogen cells and their structural characteristics in susceptibility and resistance to excoriation in immature and mature potato tuber (Solanum tuberosum L.) periderm. Ann Bot 88:555–561
Mackintosh RW, Davies SP, Clarke PR, Weekes J, Gillespie JG, Gibb BJ, Hardie DG (1992) Evidence for a protein kinase cascade in higher plants. 3-Hydroxy-3-methylglutaryl-CoA reductase kinase. Eur J Biochem 209:923–931
McCue KF, Shepherd LVT, Allen PV, Maccree MM, Rockhold DR, Corsini DL, Davies HV, Belknap WR (2005) Metabolic compensation of steroidal glycoalkaloid biosynthesis in transgenic potato tubers: using reverse genetics to confirm the in vivo enzyme function of a steroidal alkaloid galactosyltransferase. Plant Sci 168:267–273
McCue KF, Allen PV, Shepherd LVT, Blake A, Whitworth J, Maccree MM, Rockhold DR, Stewart D, Davies HV, Belknap WR (2006) The primary in vivo steroidal alkaloid glucosyltransferase from potato. Phytochemistry 67:1590–1597
McCue KF, Allen PV, Shepherd LVT, Blake A, Maccree MM, Rockhold DR, Novy RG, Stewart D, Davies HV, Belknap WR (2007) Potato glycosterol rhamnosyltransferase, the terminal step in triose side-chain biosynthesis. Phytochemistry 68:327–334
Moehs CP, Allen PV, Friedman M, Belknap WR (1997) Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J 11:227–236
Morgan MRA, Coxon DT, Bramham S, Chan HWS, Vangelde WMJ, Allison MJ (1985) Determination of the glycoalkaloid content of potato tubers by 3 methods including enzyme linked immunosorbent assay. J Sci Food Agri 36:282–288
Osman S, Sinden SL, Deahl K, Moreau R (1987) The metabolism of solanidine by microsomal fractions from Solanum chacoense. Phytochemistry 26:3163–3165
Pehu E, Gibson RW, Jones MGK, Karp A (1990) Studies on the genetic basis of resistance to potato leaf roll virus, potato virus Y and potato virus X in Solanum brevidens using somatic hybrids of Solanum brevidens and Solanum tuberosum. Plant Sci 69:95–101
Percival G, Dixon G, Sword A (1994) Glycoalkaloid concentration of potato tubers following continuous illumination. J Sci Food Agric 66:139–144
Percival GC, Karim MS, Dixon GR (1998) Influence of light enhanced glycoalkaloids on resistance of potato tubers to Fusarium sulphureum and Fusarium solani var. coeruleum. Plant Pathol 47:665–670
Rangarajan A, Miller AR, Veilleux RE (2000) Leptine glycoalkaloids reduce feeding by Colorado potato beetle in diploid Solanum sp hybrids. J Am Soc Hortic Sci 125:689–693
Reeve RM, Hautala E, Weaver ML (1969) Anatomy and compositional variation within potatoes 1. Developmental histology of the tuber. Am Potato J 46:361–373
Rokka V-M, Xu Y-S, Kankila J, Kuusela A, Pulli S, Pehu E (1994) Identification of somatic hybrids of dihaploid Solanum tuberosum lines and S. brevidens by species specific RAPD patterns and assessment of disease resistance of the hybrids. Euphytica 80:207
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, New York
Sanford LL, Deahl KL, Sinden SL, Ladd TL (1992) Glycoalkaloid contents in tubers from Solanum tuberosum populations selected for potato leafhopper resistance. Am Potato J 69:693–703
Sanford LL, Kobayashi RS, Deahl KL, Sinden SL (1997) Diploid and tetraploid Solanum chacoense genotypes that synthesize leptine glycoalkaloids and deter feeding by Colorado potato beetle. Am Potato J 74:15–21
Shih M, Kuc J (1973) Incorporation of C-14 from acetate and mevalonate into rishitin and steroid glycoalkaloids by potato tuber slices inoculated with Phytophthora infestans. Phytopathology 63:826–829
Sinden SL, Sanford LL, Deahl KL (1986) Segregation leptine glycoalkaloids in Solanum chacoense bitter. J Agric Food Chem 34:372–377
Sinden SL, Sanford LL, Cantelo WW, Deahl KL (1988) Bioassay of segregating plants. A strategy for studying chemical defence. J Chem Ecol 14:1941–1950
Smith DB, Roddick JG, Jones JL (1996) Potato glycoalkaloids: some unanswered questions. Trends Food Sci Tech 7:126–131
Stermer BA, Bostock RM (1987) Involvement of 3-hydroxy-3-methylglutaryl coenzyme-A reductase in the regulation of sesquiterpenoid phytoalexin synthesis in potato. Plant Physiol 84:404–408
Stermer BA, Bianchini GM, Korth KL (1994) Regulation of HMG-CoA reductase activity in plants. J Lipid Res 35:1133–1140
Threlfall DR, Whitehead IM (1988) Coordinated inhibition of squalene synthetase and induction of enzymes of sesquiterpenoid phytoalexin biosynthesis in cultures of Nicotiana tabacum. Phytochemistry 27:2567–2580
Valkonen JPT, Keskitalo M, Vasara T, Pietila L (1996) Potato glycoalkaloids: a burden or a blessing? Crit Rev Plant Sci 15:1–20
Veilleux RE, Miller AR (1998) Hybrid breakdown in the F-1, between Solanum chacoense and S. phureja and gene transfer for leptine biosynthesis. J Am Soc Hortic Sci 123:854–858
Vogeli U, Chappell J (1988) Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol 88:1291–1296
Yang ZB, Park HS, Lacy GH, Cramer CL (1991) Differential activation of potato 3-hydroxy-3-methylglutaryl coenzyme-A reductase genes by wounding and pathogen challenge. Plant Cell 3:397–405
Yoshioka H, Yamada N, Doke N (1999) cDNA cloning of sesquiterpene cyclase and squalene synthase, and expression of the genes in potato tuber infected with Phytophthora infestans. Plant Cell Physiol 40:993–998
Zook MN, Kuc JA (1991) Induction of sesquiterpene cyclase and suppression of squalene synthetase activity in elicitor treated or fungal infected potato tuber tissue. Physiol Mol Plant Pathol 39:377–390
Acknowledgment
This work is a contribution of the Volcani Center no. 129/2006.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krits, P., Fogelman, E. & Ginzberg, I. Potato steroidal glycoalkaloid levels and the expression of key isoprenoid metabolic genes. Planta 227, 143–150 (2007). https://doi.org/10.1007/s00425-007-0602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0602-3