Abstract
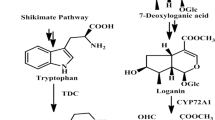
The potent anticancer and antiviral compound camptothecin (CPT) is a monoterpene indole alkaloid produced by Camptotheca acuminata. In order to investigate the biosynthetic pathway of CPT, we studied the early indole pathway, a junction between primary and secondary metabolism, which generates tryptophan for both protein synthesis and indole alkaloid production. We cloned and characterized the alpha subunit of anthranilate synthase (ASA) from Camptotheca (designated CaASA), catalyzing the first committed reaction of the indole pathway. CaASA is encoded by a highly conserved gene family in Camptotheca. The two CaASA genes are differentially regulated. The level of CaASA2 is constitutively low in Camptotheca and was found mainly in the reproductive tissues in transgenic tobacco plants carrying the CaASA2 promoter and β-glucuronidase gene fusion. CaASA1 was detected to varying degrees in all Camptotheca organs examined and transiently induced to a higher level during seedling development. The spatial and developmental regulation of CaASA1 paralleled that of the previously characterized Camptotheca gene encoding the beta subunit of tryptophan synthase as well as the accumulation of CPT. These data suggest that CaASA1, rather than CaASA2, is responsible for synthesizing precursors for CPT biosynthesis in Camptotheca and that the early indole pathway and CPT biosynthesis are coordinately regulated.





Similar content being viewed by others
Abbreviations
- ASA:
-
Anthranilate synthase alpha
- CPT:
-
Camptothecin
- DPI:
-
Days post imbibition
- GUS:
-
β-Glucuronidase
- TDC:
-
Tryptophan decarboxylase
- TSB:
-
Tryptophan synthase beta subunit
References
Berlyn MB, Last RL, Fink GR (1989) A gene encoding the tryptophan synthase beta subunit of Arabidopsis thaliana. Proc Natl Acad Sci USA 86:4604–4608
Bohlmann J, DeLuca V, Eilert U, Martin W (1995) Purification and cDNA cloning of anthranilate synthase from Ruta graveolens: modes of expression and properties of native and recombinant enzymes. Plant J 7:491–501
Bohlmann J, Lins T, Martin W, Eilert U (1996) Anthranilate synthase from Ruta graveolens. Duplicated AS alpha genes encode tryptophan-sensitive and tryptophan-insensitive isoenzymes specific to amino acid and alkaloid biosynthesis. Plant Physiol 111:507–514
Crawford IP (1989) Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol 43:567–600
Facchini PJ, Park SU (2003) Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy. Phytochemistry 64:177–186
Herrmann KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503
Hutchinson CR, Heckendorf AH, Daddona PE, Hagaman E, Wenkert E (1974) Biosynthesis of camptothecin. I. Definition of the overall pathway assisted by carbon-13 nuclear magnetic resonance analysis. J Am Chem Soc 96:5609–5611
Knaggs AR (2003) The biosynthesis of shikimate metabolites. Nat Prod Rep 20:119–136
Kreps JA, Ponappa T, Dong W, Town CD (1996) Molecular basis of alpha-methyltryptophan resistance in amt-1, a mutant of Arabidopsis thaliana with altered tryptophan metabolism. Plant Physiol 110:1159–1165
Kutchan TM (1993) Strictosidine: from alkaloid to enzyme to gene. Phytochemistry 32:493–506
Li J, Last RL (1996) The Arabidopsis thaliana trp5 mutant has a feedback-resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol 110(1):51–59
Li J, Chen S, Zhu L, Last RL (1995a) Isolation of cDNAs encoding the tryptophan pathway enzyme indole-3-glycerol phosphate synthase from Arabidopsis thaliana. Plant Physiol 108:877–878
Li J, Zhao J, Rose AB, Schmidt R, Last RL (1995b) Arabidopsis phosphoribosylanthranilate isomerase: molecular genetic analysis of triplicate tryptophan pathway genes. Plant Cell 7:447–461
López-Meyer M, Nessler CL (1997) Tryptophan decarboxylase is encoded by two autonomously regulated genes in Camptotheca acuminata which are differentially expressed during development and stress. Plant J 11:1167–1175
López-Meyer M, Nessler CL, McKnight TD (1994) Sites of accumulation of the antitumor alkaloid camptothecin in Camptotheca acuminata. Planta Med 60:558–560
Lorence L, Nessler CL (2004) Camptothecin, over four decades of surprising findings. Phytochemistry 65:2735–2749
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu H, McKnight TD (1999) Tissue-specific expression of the beta-subunit of tryptophan synthase in Camptotheca acuminata, an indole alkaloid-producing plant. Plant Physiol 120:43–52
Matsuo H, Taniguchi K, Hiramoto T, Yamada T, Ichinose Y, Toyoda K, Takeda K, Shiraishi T (2001) Gramine increase associated with rapid and transient systemic resistance in barley seedlings induced by mechanical and biological stresses. Plant Cell Physiol 42:1103–1111
McKnight TD, Bergey DR, Burnett RJ, Nessler CL (1991) Expression of enzymatically active and correctly targeted strictosidine synthase in transgenic tobacco plants. Planta (Heidelberg) 185:148–152
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nagao RT, Shah DM, Eckenrode VK, Meagher RB (1981) Multigene family of actin-related sequences isolated from a soybean genomic library. DNA 1:1–9
Niyogi KK, Fink GR (1992) Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell 4:721–733
Oberlies NH, Kroll DJ (2004) Camptothecin and taxol: historic achievements in natural products research. J Nat Prod 67:129–135
Radwanski ER, Zhao J, Last RL (1995) Arabidopsis thaliana tryptophan synthase alpha: gene cloning, expression, and subunit interaction. Mol Gen Genet 248:657–667
Rothenberg ML (1997) Topoisomerase I inhibitors: review and update. Ann Oncol 8:837–855
Soepenberg O, Sparreboom A, Verweij J (2003) Clinical studies of camptothecin and derivatives. Alkaloids Chem Biol 60:1–50
Song HS, Brotherton JE, Gonzales RA, Widholm JM (1998) Tissue culture-specific expression of a naturally occurring tobacco feedback-insensitive anthranilate synthase. Plant Physiol 117:533–543
Tozawa Y, Hasegawa H, Terakawa T, Wakasa K (2001) Characterization of rice anthranilate synthase alpha-subunit genes OASA1 and OASA2. Tryptophan accumulation in transgenic rice expressing a feedback-insensitive mutant of OASA1. Plant Physiol 126:1493–1506
Vazquez-Flota FA, St-Pierre B, De Luca V (2000) Light activation of vindoline biosynthesis does not require cytomorphogenesis in Catharanthus roseus seedlings. Phytochemistry 55:531–536
Vidensek N, Lim P, Campbell A, Carlson C (1990) Taxol content in bark, wood, root, leaf, twig, and seedling from several Taxus species. J Nat Prod 53:1609–1610
Wall ME (1998) Camptothecin and taxol: discovery to clinic. Med Res Rev 18:299–314
Yamazaki Y, Sudo H, Yamazaki M, Aimi N, Saito K (2003) Camptothecin biosynthetic genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol 44:395–403
Zhao J, Last RL (1995) Immunological characterization and chloroplast localization of the tryptophan biosynthetic enzymes of the flowering plant Arabidopsis thaliana. J Biol Chem 270:6081–6087
Zhou X, Wang W, Hu T (1992) Determination of total alkaloid in seedling bulbs of Fritillaria thunbergii Miq. Zhongguo Zhong Yao Za Zhi 17:270–272, 320
Acknowledgements
We thank members of McKnight laboratory for their helpful discussion and assistance during the course of this work and Craig Nessler and Argelia Lorence for critically reading the manuscript. This work was supported by a grant from the National Institutes of Health (R01-CA-57592).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, H., Gorman, E. & McKnight, T.D. Molecular characterization of two anthranilate synthase alpha subunit genes in Camptotheca acuminata. Planta 221, 352–360 (2005). https://doi.org/10.1007/s00425-004-1450-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1450-z