Abstract
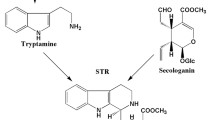
Nothapodytes foetida, an endangered tree of Indian origin, is a major source of the anti-cancer monoterpenoid indole alkaloid, camptothecin (CPT). Strictosidine synthase (STR) condenses tryptamine and secologanin to form strictosidine, a universal precursor of terpenoid indole alkaloids including CPT. We cloned full-length str cDNA with an open reading frame of 1059 bp from N. foetida (Nfstr) using a homology-based approach. Different tissues of N. foetida from in vitro grown cultures, as well as a mature tree, showed expression of STR, confirming the constitutive nature of the gene. In vitro tissues showed a positive correlation between STR expression and the CPT content, but tissues from wild-type mature plants did not show a similar pattern. Transgenic Ophiorrhiza rugosa plants overexpressing Nfstr showed 1.9-fold higher CPT than non-transformed plants. The results indicated that overexpression of Nfstr in target plants could improve the levels of CPT and may provide an alternative and sustainable source of camptothecin.
Key message
We report the full-length sequence and expression analysis of strictosidine synthase cDNA from Nothapodytes foetida (Nfstr). Further, the overexpression of Nfstr in Ophiorrhiza resulted in twofold enhancement in camptothecin levels.







Similar content being viewed by others
Abbreviations
- CPT:
-
Camptothecin
- dw:
-
Dry weight
- TIA:
-
Terpenoid indole alkaloid
- STR:
-
Strictosidine synthase
References
Aerts RJ, Van der Leer T, Van der Heijden R, Verpoorte R (1990) Developmental regulation of alkaloid production in Cinchona seedlings. J Plant Physiol 13:86–91
Aerts RJ, De Waal A, Pennings EJM, Verpoorte R (1992) The distribution of strictosidine synthase activity and alkaloids in Cinchona plants. Planta 183:536–541
Aerts RJ, Gisi D, De Carolis E, De Luca V, Baumann TW (1994) Methyl jasmonate vapour increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J 5:635–643
Aiyama R, Nagai H, Nokata K, Shinohara C, Swada SA (1988) Camptothecin derivative from Nothapodytes foetida. Phytochemistry 27:3663–3664
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bernonville TD, Clastre M, Besseau S, Oudin A, Burlat V, Glévarec G, Lanoue A, Papon N, Giglioli-Guivarc’h N, St-Pierre B, Courdavault V (2015) Phytochemical genomics of the Madagascar periwinkle: unravelling the last twists of the alkaloid engine. Phytochemistry 113:9–23
Bodley AL, Cumming JN, Shapiro TA (1998) Effects of camptothecin, a topoisomerase I inhibitor, on Plasmodium falciparum. Biochem Pharmacol 55:709–711
Bustin SA, BenesV GJA, Hellemans J, Hugget J, Kubista M, Mueller R, Nolan T, Pfaffi MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Canel C, Lopes-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JH, Verpoorte R (1998) Effect of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Cui L, Ni X, Ji Q, Teng X, Yang Y, Wu C, Zekria D, Zhang D, Kai G (2015) Co-overexpression of geraniol-10-hydroxylase and strictosidine synthase improves anti-cancer drug camptothecin accumulation in Ophiorrhiza pumila. Sci Rep 5:8227
Deepthi S, Satheeshkumar K (2016) Enhanced camptothecin production induced by elicitors in the cell suspension cultures of Ophiorrhiza mungos Linn. Plant Cell Tissue Organ Cult 124:483–493
Dellaporta J, Wood JB, Hicks A (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 14:19–21
Fulzele DP, Sadive RS (2003) Somatic embryogenesis, plant regeneration and the evaluation of camptothecin content in Nothapodytes foetida. Vitro Cell Dev Biol Plant 39:212–216
Fulzele DP, Satdive RK (2005) Distribution of anticancer drug camptothecin in Nothapodytes foetida. Fitoterapia 76:643–648
Fulzele DP, Satdive R, Kamble S, Singh S, Singh S (2015) Improvement of anticancer drug camptothecin production by gamma irradiation on callus cultures of Nothapodytes foetida. Int. J Pharma Res Allied Sci 4:19–27
Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RTA (1985) Simple and general method of transferring genes into plants. Science 227:1229–1231
Huang HC, Sung PH, Do YY, Huang PL (2012) Differential expression and functional characterization of the NADPH cytochrome P450 reductase genes from Nothapodytes foetida. Plant Sci 190:16–23
Isah T, Mujib A (2015) In vitro propagation and camptothecin production in Nothapodytes nimmoniana. Plant Cell Tissue Organ Cult 121:1–10
Kutchan TM, Hampp N, Lottspeich F, Beyreuther K, Zenk MH (1988) The cDNA clone for strictosidine synthase from Rauvolfia serpentine. DNA sequence determination and expression in Escherichia coli. FEBS Lett 237:40–44
Lopez-Meyer M, Nessler CL, Mcknight TD (1994) Sites of accumulation of the antitumor alkaloid camptothecin in Camptotheca acuminate. Planta Med 60:558–560
Lu Y, Wang H, Wang W, Zhongying Q, Li L, Wang J, Zhou G, Kai G (2009) Molecular characterization and expression analysis of a new cDNA encoding strictosidine synthase from Ophiorrhiza japonica. Mol Biol Rep 36:1845–1852
Manjunatha BL, Singh HR, Ravikanth G, Nataraja KN, Shankar R, Kumar S, Uma Shaanker R (2016) Transcriptome analysis of stem wood of Nothapodytes nimmoniana (Graham) Mabb. Identifies genes associated with biosynthesis of camptothecin, an anti-carcinogenic molecule. J Biosci 41(1):119–131
Martin KP, Zhang CL, Hembrom ME, Slater A, Madassery J (2008) Adventitious root induction in Ophiorrhiza prostrata: a tool for the production of camptothecin (an anticancer drug) and rapid propagation. Plant Biotechnol Rep 2:163–169
McKnight TD, Roessner CA, Devagupta R, Scott AI, Nessler CL (1990) Nucleotide sequence of a cDNA encoding the vacuolar protein strictosidine synthase from Catharanthus roseus. Nucleic Acids Res 18:4939
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Namdeo AG, Sharma A (2012) HPLC analysis of camptothecin content in various parts of Nothapodytes foetida collected on different periods. Asian Pac J Trop Biomed 2:389–393
Pan Q, Wang Q, Yuan F, Xing S, Zhao J, Choi YH, Verpoorte R, Tian Y, Wang G, Tang K (2012) Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PLoS ONE 7(8):e43038
Priel E, Showwalter SD, Blair DG (1991) Inhibition of human immunodeficiency virus (HIV-1) replication in vitro by noncytotoxic doses of camptothecin, a topoisomerase I inhibitor. AIDS Res Hum Retrovir 7(1):65–72
Rather GA, Sharma A, Pandith SA, Kaul V, Nandi U, Misra P, Lattoo SK (2018) De novo transcriptomic analyses reveals putative pathway genes involved in biosynthesis and regulation of camptothecin in Nothapodytes nimmoniana (Graham) Mabb. Plant Mol Biol 96:197–215
Roja G (2008) Micropropagation and production of camptothecin from in vitro plants of Ophiorrhiza rugosa var. decumbens. Nat Prod Res 22:1017–1023
Ruan J, Zhang J, Li M, Zhu Y, Sun L, Jin H, Su H, Xu M (2014) Dependence of UV-B-induced camptothecin production on nitrate reductase-mediated nitric oxide signaling in Camptotheca acuminata suspension cell cultures. Plant Cell Tissue Organ Cult 118:269–278
Sakuta M, Komamine A (1987) Cell growth and accumulation of secondary metabolites. In: Constabel F, Vasil IK (eds) Cell culture and somatic cell genetics of plats. Cell culture in phytochemistry, vol 4. Academic Press, San Diego, pp 49–76
Shivaprakash KN, Ramesha BT, Shaanker RU, Dayanandan S, Ravikanth G (2014) Genetic structure, diversity and long term viability of a medicinal plant, Nothapodytes nimmoniana Graham. (Icacinaceae), in protected and non-protected areas in the Western Ghats biodiversity hotspot. PLoS ONE 9:e112769
Sibéril Y, Benhamron S, Memelink J, Giglioli-Guivarc’h N, Thiersault M, Boissoni B, Doireau P, Gantet P (2001) Catharanthus roseus G-box binding factors 1 and 2 act as repressors of strictosidine synthase gene expression in cell cultures. Plant Mol Biol 45:477–488
Singh S, Premsagar K, Ramachandran V, Eapen S (2011) Transgenic Nicotiana tabacum plants expressing a fungal copper transporter gene show enhanced acquisition of copper. Plant Cell Rep 30:1929–1938
Stöckigt J, Ruppert M (1999) Strictosidine—the biosynthetic key to monoterpenoid indole alkaloid. In: Barton DHR, Nakanishi K, Meth-Cohn O, Kelly JW (eds) Comprehensive natural products chemistry: amino acids, peptides, porphyrins and alkaloids, vol 4. Elsevier, Amsterdam, pp 109–139
Stöckigt J, Zenx MH (1977) Strictosidine (Isovincoside): the key intermediate in the biosynthesis of monoterpenoid indole alkaloids. J Chem Soc Chem Commun. https://doi.org/10.1039/C39770000646
Sun Y, Luo H, Sun YLC, Song J, Niu Y, Zhu Y, Dong L, Lv A, Tramontano E, Chen S (2011) Pyrosequencing of Camptotheca acuminata transcriptome reveals putative genes involved in camptothecin biosynthesis and transport. BMC Genom 12:533
Tamura K, Stecher G, Peterson D, Filipski A, Sudhir Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Uma Shaanker R, Ramesha BT, Ravikanth G, Gunaga R, Vasudeva R, Ganeshaiah KN (2008) Chemical profiling of Nothapodytes nimmoniana for camptothecin, an important anticancer alkaloid: toward the development of a sustainable production system. In: Ramawat KG, Merillon JM (eds) Bioactive molecules and medicinal plants. Springer, London, pp 197–213
Venditto VJ, Simanek EE (2010) Cancer therapies utilizing the camptothecin: a review of the in vivo literature. Mol Pharm 7:307–349
Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA (1966) Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloid leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 88(16):3888–3890
Yamazaki Y, Urano A, Sudo H, Kitajima M, Takayama H, Yamazaki M, Aimi N, Saito K (2003) Metabolite profiling of alkaloids and strictosidine synthase activity in camptothecin producing plants. Phytochemistry 62:461–470
Acknowledgements
Work communicated in the present manuscript is supported by Department of Atomic Energy, Government of India. Authors thank Head, NABTD for his encouragement and support.
Author information
Authors and Affiliations
Contributions
SS conceived and designed the study. SS, SNK, and RKS performed the experiments and analyzed the data. SS, SNK, RKS, and DPF contributed inputs, wrote and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, S., Kamble, S.N., Satdive, R.K. et al. Heterologous overexpression of Nothapodytes foetida strictosidine synthase enhances levels of anti-cancer compound camptothecin in Ophiorrhiza rugosa. Plant Cell Tiss Organ Cult 141, 67–76 (2020). https://doi.org/10.1007/s11240-020-01767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01767-9