Abstract
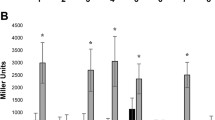
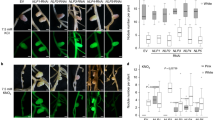
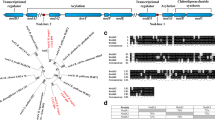
Plant genes that are induced during the formation and function of a root nodule are called nodulin genes. Cloning and functional analysis of nodule-specific gene products are of valuable help in establishing the role and requirements of the host plant for the specificity and effectiveness of the symbiosis. A cDNA clone (nod22) was isolated from Phaseolus vulgaris L. (common bean) cDNA library derived from Rhizobium -infected roots. Nodulin 22 (Nod22) transcripts are accumulated from early to late stages in root nodule development. RT-PCR in situ studies indicated that Nod22 transcripts are highly accumulated in cortical, vascular bundle and infected cells. The deduced Nod22 protein contains a highly hydrophobic N-terminus, with signal peptide characteristics, and a C-terminal extension with high identity to the α-crystallin domains found in α-crystallin lens chaperone, and other small heat-shock proteins. These domains have not been previously described in other known nodulins, but have been observed in small heat-shock proteins found in plant tissues exposed to elevated temperature and oxidative stress. Nod22, when it is over-expressed in Escherichia coli, cells confers protection against oxidative stress suggesting its possible role in plant host protection from oxidative toxicity during the Rhizobium-legume symbiosis.






Similar content being viewed by others
Abbreviations
- ACD :
-
α-Crystallin domain
- CLSM :
-
Confocal laser scanning microscopy
- sHsp :
-
Small heat-shock protein
- SP :
-
Signal peptide
References
Andley UP, Harendra C Patel, Jing-Hua Xi (2002) The R116C mutation in αA-crystallin diminishes its protective ability against stress-induced Lens epithelial cell apoptosis. J Biol Chem 277:10178–10186
Becana M, Poaris FJ, Sandalio LM, Del Río LA (1989) Isoenzymes of superoxide dismutase in nodules of Phaseolus vulgaris L., Pisum sativum L., and Vigna ungiculata (L). Plant Physiol 90:1286–1292
Capote N, Sánchez F (1997) Characterization of common bean uricase II and its expression in organs other than nodules. Plant Physiol 115:1307–1317
Cook D, Dreyer D, Bonnet D, Howell M, Nony E, Vandenbosch K (1995) Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 7:43–55
Dantan E, Rosenstein Y, Quinto C, Sánchez F (2001) Actin monoubiquitylation is induced in plants in response to pathogens and symbionts. MPMI 14:1267–1273
Derham BK, Ellory JC, Bron AJ, Harding JJ (2003) The molecular chaperone α-crystallin incorporated into red cell ghosts protects membrane Na/K-ATPase against glycation and oxidative stress. Eur J Biochem 270:2605–2611
Echey-Kaltenbach H, Keifer E, Grosskog E, Ernst D, Sandermann H Jr (1997) Differential transcript induction of parsley pathogenesis-related proteins and and a small heat-shock protein by ozone and heat shock. Plant Mol Biol 33:343–350
Foreman J, Demidchick V, Bothwell JHF, Mylona P, Maidema H, Torres MA, Linstead P, Costa S, Browlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Guillén G, Valdés-López V, Noguez R, Olivares J, Rodríguez-Zapata LC, Pérez H, Vidali L, Villanueva MA, Sánchez F (1999) Profilin in Phaseolus vulgaris is encoded by two genes (only one expressed in root nodules) but multiple isoforms are generated in vivo by phosphorylation on tyrosine residues. Plant J 19:497–508
Huckelhoven R, Kogel K-H (2003) Reactive oxygen intermediates in plant-microbe interactions: who is who in powdery mildew resistance. Planta 216:891–902
Iturbe-Ormaetxe I, Matamoros MA, Ubio MC, Dalton DA, Becana M (2001) The andioxidants of legume nodule mitochondira. MPMI 14:1189–1196
Joe MK, Park SM, Lee YS, Hwang DS, Hong CB (2000) High temperature stress resistance of Escherichia coli induced by a tobacco class I low molecular weight heat-shock protein. Mol Cells 10:519–524
Jofré A, Molinas M, Pla M (2003) A 10-kDa class-CI sHsp protects E. coli from oxidative and high-temperature stress. Planta 217:813–819
Jong WW de, Caspers GJ, Leunissen JA (1998) Geneology of the α-crystallin small heat-shock protein superfamily. Int J Biol Macromol 22:151–162
Kelley LA, MacCallum RM, Sternberg MJE (2000) Enhanced genome annotation using structural profiles in the program 3D-PSSM. J Mol Biol 299:501–522
Kyte J, Doollittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Maniatis T, Fritsch EF, Sambrook J (1989) Molecular cloning: laboratory manual. Cold Spring Harbour Press, N.Y.
Møller IM (2001) Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Montfort RL van, Basha E, Friedrich KL, Slingsby C, Vierling E (2001) Crystal structure and assembly of a eukaryotic small heat-shock protein. Nat Struct Biol 8:1025–1030
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ (1999) Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Dev Cell Biol 126:4405–4419
Sabatini DD, Kreibich G, Morimoto T, Adesnik M (1982) Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol 92:1–22
Sánchez F, Padilla JE, Pérez H, Lara M (1991) Control of nodulin genes in root-nodule development and metabolism. Annu Rev Plant Physiol Plant Mol Biol 42:507–528
Santos R, Herouart D, Puppo A, Touati D (2000) Critical protective role of bacterial superoxide dismutase in Rhizobium-legume symbiosis. Mol Microbiol 38:750–759
Scharf KD, Siddique M, Vierling E (2001) The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins). Cell Stress Chaperones 6:225–237
Shaw SL, Long SR (2003) Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol 132:2196–2204
Soto A, Allona I, Collada C, Guevara M, Casado R, Rodriguez-Cerezo E, Arangocillo C, Gómez L (1999) Heterologous expresión of a plant small heat-shock protein enhances Escherichia coli viability under heat and cold stress. Plant Physiol 120:521–528
Stougaard J (2000) Regulators and regulations of legume root nodule development. Plant Physiol 124:531–540
Stromer T, Ehrnsperger M, Gaestel M, Buchner J (2003) Analysis of the interaction of small heat-shock proteins with unfolding proteins. J Biol Chem 278:18015–18021
Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M (1998) A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20:92–95
Wang K, Spector A (1996) A alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem 242:56–66
Waters ER (1995) Molecular evolution of the small heat-shock proteins in plants. Genetics 141:785–795
Waters ER, Lee GJ, Vierling E (1996) Evolution, structures and function of the small heat-shock proteins in plants. J Exp Bot 47:325–338
Whitham SA, Anderberg RJ, Chrisholm ST, Carrington JC (2000) Arabidopsis RTM2 gene is necessary for specific restriction of tobacco etch virus and encodes an usual small heat-shock-like protein. Plant Cell 12:569–582
Worley KC, Wiese BA, Smith RF (1995) BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genome Res 5:173–84
Yeh KW, Jinn TL, Yeh CH, Chen YM, Lin CY (1994) Plant low-molecular-mass heat-shock proteins: their relationships to the acquisition of thermotolerance in plants. Biotechnol Appl Biochem 19:41–49
Yeh CH, Chen YM, Lin CY (2002) Functional regions of rice heat-shock protein Oshp16.9, required for conferring thermotolerance in Escherichia coli. Plant Physiol 128:661–668
Zhang X, Zhang L, Fong F, Gao J, Galbraith DW; Song CP (2001) Hydrogen peroxide is envolved in abscisic acid stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Acknowledgements
Assistance by Noreide Nava and Gabriel Guillén is appreciated. We gratefully acknowledge Xóchitl Alvarado for the confocal laser scanning microscopy, and Nayeli Sánchez for processing the images in Adobe Photoshop. Dr Otto Gieger’s critical review of the manuscript is appreciated. This research was funded by projects DGAPA (IN232002), and CONACYT (33350-N).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad, A., Miranda-Ríos, J., Estrada Navarrete, G. et al. Nodulin 22 from Phaseolus vulgaris protects Escherichia coli cells from oxidative stress. Planta 219, 993–1002 (2004). https://doi.org/10.1007/s00425-004-1303-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1303-9