Abstract
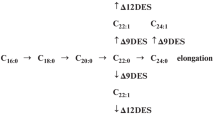
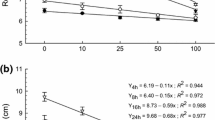
The monoterpenes 1,8-cineole, thymol, geraniol, menthol and camphor strongly inhibited the root growth of Zea mays L. seedlings. They induced an oxidative stress as measured by the production of malondialdehyde, conjugated dienes and peroxides. This oxidative stress depended on the length of the exposure and on the monoterpene applied. The total fatty acid content was measured and fatty acid composition was analyzed. Unsaturated fatty acids increased in the treated samples. The alcoholic and non-alcoholic monoterpenes appeared to have different modes of action.


Similar content being viewed by others
Abbreviations
- MDA :
-
Malondialdehyde
- TFA :
-
Total fatty acid content
- FA :
-
Fatty acid
- IC 80 :
-
Concentration causing 80% inhibition
References
Abrahim D, Braguini WL, Kelmer-Bracht AM, Ishii-iwamoto EL (2000) Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J Chem Ecol 26:611–624
Asplund RO (1968) Monoterpenes: relationship between structure and inhibition of germination. Phytochemistry 7:1995–1997
Beuge JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Boveris A, Cadenas E, Chance B (1980) Low level chemiluminescence of the lipoxygenase reaction. Photobiochem Photobiophys 1:175–182
Bradow JM, Connick WJ Jr (1988) Volatile methyl ketone seed-germination inhibitors from Amaranthus palmeri S. Wats. residues. J Chem Ecol 14:1617–1631
Bradow JM, Connick WJ Jr (1990) Volatile seed germination inhibitors from plant residues. J Chem Ecol 16:645–666
Chapman RA, MacKay K (1949) The estimation of peroxides in fats and oils by the ferric thiocyanate method. J Am Oil Chem Soc 7:360–363
Deighton N, Glidewell SM, Deans SG, Goodman BA (1993) Identification by EPR spectroscopy of carvacrol and thymol as the mayor sources of free radicals in the oxidation of plant essential oils. J Sci Food Agric 63:221–225
De Santis A, Landi P, Genchi G (1999) Changes of mitochondrial properties in maize seedlings associated with selection for germination at low temperature. Fatty acid composition, cytochrome c oxidase, and adenine nucleotide translocase activities. Plant Physiol 119:743–754
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dudai N, Larkov O, Mayer AM, Poljakoff-Mayber A, Putievsky E, Lerner HR (2000) Metabolism of essential oils during inhibition of wheat seed germination. In: Black M, Bradford KJ, Vázquez-Ramos J (eds) Seed biology: advances and applications. CABI, Wallingford, UK, p 315
Einhellig FA, Leather GR (1988) Potentials for exploiting allelopathy to enhance crop production. J Chem Ecol 14:1829–1844
Fischer NH (1986) The function of mono and sesquiterpenes as plant germination and growth regulators. In: Putnam A, Chung-Shih Tang (eds) The science of allelopathy. Wiley, London, p 203
Fischer NH (1991) Plant terpenoids as allelopathic agents. In: Harborne JB, Tomas-Barberan FA (eds) Ecological chemistry and biochemistry of plant terpenoids. Clarenden, Oxford, p 387
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Fridovich I (1986) Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 58:61–97
García DA, Perillo MA, Zygadlo JA, Martijena ID (1995) The essential oil from Tagetes minuta L. modulates binding of [3H]-FNTZ to membranes from brain cortex. Lipids 30:1105–1110
Grosso NR, Lamarque AL, Maestri DM, Zygadlo JA, Guzman CA (1994) Fatty acid variation of runner peanut (Arachis hypogaea L.) among geographic from Córdoba (Argentina). J Am Oil Chem Soc 71:541–542
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 188–275
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acids peroxidation. Arch Biochem Biophys 125:189–198
Hooks MA, Bode K, Couée I (1995) Regulation of acyl-CoA oxidases in maize seedlings. Phytochemistry 40:657–660
Jiménez A, Hernández JA, Pastori G, del Rio LA, Sevilla F (1998) Role of the ascorbate–glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol 118:1327–1335
Karp G (ed) (1987) Biología Celular. McGraw-Hill, Maidenhead, UK
Koga T, Nagao A, Terao J, Sawada K, Mukai K (1994) Synthesis of a phosphatidyl derivative of vitamin E and its antioxidant activity in phospholipid bilayers. Lipids 29:83–88
Koitabashi R, Suzuki T, Kawazu T, Sakai A, Kuroiwa H, Kuroiwa T (1997) 1,8-Cineole inhibits root growth and DNA synthesis in the root apical meristem of Brassica campestris L.. J Plant Res 110:1–6
Kosugi H, Jojima T, Kikugawa K (1989) Thiobarbituric acid-reactive substances from peroxidized lipids. Lipids 24:873–881
Lorber P, Muller WH (1976) Volatile growth inhibitors produced by Salvia leucophylla: effects on seedling root tip ultrastructure. A J Bot 63:196–200
Maffei M, Camusso W, Sacco S (2001) Effect of Mentha × piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 58:703–707
McKersie BD, Hoekstra FA, Krieg LC (1990) Differences in the susceptibility of plant membrane lipids to peroxidation. Biochim Biophys Acta 1030:119–126
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Muscari CL, Frascaro M, Guarnieri C, Caldarera CIM (1990) Mitochondrial function and superoxide generation from submitochondrial particles of aged rat hearts. Biochim Biophys Acta 1015:200–204
Panasiuk O, Bills DD, Leather GR (1986) Allelopathic influence of Sorghum bicolor on weeds during germination and early development of seedlings. J Chem Ecol 12:1533–1543
Pastori GM, del Rio LA (1994) An activated-oxygen-mediated role for peroxisomes in the mechanism of senescence of Pisum sativum L. leaves. Planta 193:385–391
Pastori GM, Trippi VS (1995) Fatty acid composition in water- and oxygen-stressed leaves of maize and wheat strains. Phytochemistry 40:45–48
Perillo MA, Guidoti A, Costa E, Yu R, Maggio B (1994) Modulation of phospholipase A2 and C activities against dilauroylphosphorylcholine in mixed monolayers with semisynthetic derivatives of ganglioside and sphingosine. Mol Membr Biol 11:119–126
Perillo MA, Garcia DA, Marin RH, Zygadlo JA (1999) Tagetone modulates the coupling of flunitrazepam and GABA binding sites at GABAA receptor from chick brain membranes. Mol Membr Biol 16:189–194
Poirier Y, Venbe G, Caldelari D (1999) Increased flow of fatty acids toward oxidation in developing seeds of Arabidopsis deficient in diacylglycerol acyltransferase activity or synthesizing medium-chain-length fatty acids. Plant Physiol 121:1359–1366
Romagni JG, Allen SN, Dayan FE (2000) Allelopathic effects of volatile cineoles on two weedy plant species. J Chem Ecol 26:303–313
Schmedes A, Holmer G (1989) A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J Am Oil Chem Soc 66:813–816
Sikkema J, De Bont JAM, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Tarayre M, Thompson JD, Escarré J, Linhart YB (1995) Intra-specific variation in the inhibitory effects of Thymus vulgaris (Labiatae) monoterpenes on seed germination. Oecologia 101:110–118
Turina AV, Perillo MA (2003) Monoterpenes affect chlorodiazepoxide–micelle interaction through micellar dipole potential modifications. Biochim Biophys Acta 1616:112–120
Vaughn SF, Spencer GF (1993) Volatile monoterpenes as potential parent structures for new herbicides. Weed Sci 41:114–119.
Vigh L, Horváth I, Horváth LI, Dudits D, Farkas T (1979) Protoplast plasmalemma fluidity of hardenes wheats correlates with frost resistance. FEBS Lett 107:291–294
Weidenhamer JD, Menelaou M, Macias FA, Fisher NH, Richardson DR, Williamson B (1994) Allelopathic potential of menthofuran monoterpenes from Calamintha ashei. J Chem Ecol 20:3345–3359
Willms JR, Salon C, Layzell DB (1999) Evidence for light-stimulated fatty acid synthesis in soybean fruit. Plant Physiol 120:1117–1128
Acknowledgements
We thank Agencia Córdoba Ciencia and S.E.C.YT.–U.N.C. for financial support, and Dr. Perillo for critical reading of the manuscript. The comments made by two anonymous referees are gratefully acknowledged. M.P.Z. is in receipt of a fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zunino, M.P., Zygadlo, J.A. Effect of monoterpenes on lipid oxidation in maize. Planta 219, 303–309 (2004). https://doi.org/10.1007/s00425-004-1216-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1216-7