Abstract
Kidneys are central in the regulation of multiple physiological functions, such as removal of metabolic wastes and toxins, maintenance of electrolyte and fluid balance, and control of pH homeostasis. In addition, kidneys participate in systemic gluconeogenesis and in the production or activation of hormones. Acid–base conditions influence all these functions concomitantly. Healthy kidneys properly coordinate a series of physiological responses in the face of acute and chronic acid–base disorders. However, injured kidneys have a reduced capacity to adapt to such challenges. Chronic kidney disease patients are an example of individuals typically exposed to chronic and progressive metabolic acidosis. Their organisms undergo a series of alterations that brake large detrimental changes in the homeostasis of several parameters, but these alterations may also operate as further drivers of kidney damage. Acid–base disorders lead not only to changes in mechanisms involved in acid–base balance maintenance, but they also affect multiple other mechanisms tightly wired to it. In this review article, we explore the basic renal activities involved in the maintenance of acid–base balance and show how they are interconnected to cell energy metabolism and other important intracellular activities. These intertwined relationships have been investigated for more than a century, but a modern conceptual organization of these events is lacking. We propose that pH homeostasis indissociably interacts with central pathways that drive progression of chronic kidney disease, such as inflammation and metabolism, independent of etiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concentration of H+ in biological fluids influences a multitude of biological activities in living beings belonging to all life domains. Protons are central to the understanding of life because they interact with multiple biological functions and structures. H+ is here a simplified notation of the actual chemical structure of the aqueous proton (whether H13O6+, H5O2+, or H9O4+, this is a debate beyond the topic of this article and covered by others [78, 103]). Proton concentration, most often represented in its logarithmic form, pH, determines the activity of enzymes, bioavailability of substances, protein conformation, electrostatic surface of proteins, and their capacity to interact with other proteins. Protons are so central to life that we “breathe” through them; the movement of protons through the mitochondrial or cell membrane is the mechanism by which multiple forms of life produce ATP. Regulation of pH is therefore essential for normal human physiology and is involved in pathophysiological processes. In humans, pH values can be lower than 1 in the gastric acid or above 8 in the pancreatic juice. However, blood pH is normally around 7.4, which protects organs from noxious consequences of largely altered proton concentrations. Lungs and kidneys are the main organs involved in the maintenance of pH homeostasis in humans. They achieve this task by dictating the elimination of acids and bases and together with bones supporting adequate levels of extracellular buffers. In other words, they control the balance of acids and bases. These are not recent notions given that the role of kidneys and lungs in the maintenance of acid–base balance was already recognized by Claude Bernard in the 1859s Leçons Sur Les Propriétés Physiologiques Et Les Altérations Pathologiques Des Liquides De L’organisme [8]. He identified that both organs transferred forms of carbonic acid between fluid compartments, which was essential to keep pH at healthy levels.
In this review, we cover in a historical perspective how kidneys contribute to acid–base balance and how disturbed acid–base conditions affect kidney health. We show that some of the most recent findings regarding the influence of pH on renal pathophysiology and metabolism relate to central topics of investigation from the end of the XIX century and most of the XX century, but with a shift in focus towards the pro-inflammatory arm of the disease, they were somehow left in the backseat in the few past decades. It is time to bring them back to the center of the debate.
How kidneys support acid–base balance
Our understanding of how kidneys support pH homeostasis is pigeonholed through the acid–base school of thought that one follows. In one of the schools, bicarbonate is the central player in how kidneys protect the organism from acid–base disorders. This conceptual framework was derived from the Henderson-Hasselbach equation, which in turn is a product of the definition of acids and bases of Brønsted and Lowry. The other framework understands that [H+] is determined by the contribution of ions whose charge is unaltered at physiological pH, also known as strong ions (i.e., Stewart’s approach [114]). Here, we describe how kidneys perform their “acid–base roles” through the bicarbonate-centered framework. While most of the content reviewed in this article can be explained under the light of the strong ion approach, several of the mechanisms described here would lack parsimony. With that said, kidneys fundamentally protect pH homeostasis via reabsorption of bicarbonate and generation of new bicarbonate. These processes are briefly summarized in this section. Kidneys reabsorb almost the entire amount of filtered bicarbonate, with ~ 70–80% of it done in the proximal tubules, ~ 10–15% in the thick ascending limb of the loop of Henle, 4–6% in the distal convoluted tubule, and the remaining in the collecting duct. In every segment, it uses the same mechanism: secretion of H+.
When H+ is moved from the intracellular space to the luminal space, it reacts with a HCO3− molecule and forms H2CO3, and in a reaction catalyzed by carbonic anhydrases, forms CO2 and H2O.
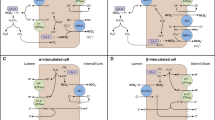
CO2 enters the cell and with H2O forms H+ and HCO3−. Therefore, the secreted H + is also formed from the same reaction, the hydration of CO2. Altogether, for every H+ secreted, a HCO3− is formed inside the cell. This bicarbonate is reabsorbed through the basolateral membrane and goes back into the bloodstream. In the proximal tubules, the movement of H+ is mostly achieved by the sodium hydrogen exchanger paralog 3 (NHE3) in the apical membrane, but also by H+ ATPase. Bicarbonate is reabsorbed mostly by the electrogenic sodium bicarbonate cotransporter 1 (NBCe1), but also by the anion exchanger 2 (AE2) in the segment 3 of the proximal tubule [19] (Fig. 1). Similar mechanisms are present all along the nephron, with changes in the protein paralogs. As an additional player, proton excretion also occurs via K+/H+ ATPase in type A intercalated cells.
Bicarbonate reabsorption and formation of new bicarbonate via ammoniagenesis in coordination with glutamine metabolism, gluconeogenesis, and activity of potassium channels in the proximal tubule. Secretion of H+ via NHE3 or H + -ATPase (not shown) leads to reabsorption of HCO3− via NBCe1 (and AE2 in the segment 3). Ammonia and HCO3− are formed from the metabolization of glutamine in the mitochondria, which provides precursors for gluconeogenesis. Glycerol and lactate are additional substrates of gluconeogenesis, but they have a minor role in response to metabolic acidosis in healthy kidneys. The transcription factor NRF2 regulates the expression of the main importer of glutamine into proximal tubular cells during acidosis, SNAT3. Potassium channels in the basolateral membrane control membrane potential impacting NBCe1 activity and ammoniagenesis. NHE3 (SLC9A3) sodium hydrogen exchanger 3, NBCe1 (SLC4A4) electrogenic sodium bicarbonate cotransporter 1, SNAT3 (Slc38a3) sodium-coupled neutral amino acid transporter 3, NRF2 (NFE2L2) nuclear factor-erythroid factor 2-related factor 2, TASK2 (KCNK5) TWIK-related acid-sensitive K( +) channel 2, KIR4.2 (KCNJ15) inward rectifier K+ channel KIR4.2, AQP7 aquaporin 7, CAII and CAIV carbonic anhydrase 2 and 4, respectively; SMCTs represent sodium-coupled monocarboxylate transporters 1 and 2 (SLC58 and SLC5A12); MCTs represent different monocarboxylate transporter members, most probably SLC16A1 and SLC16A; PDG (GLS) phosphate-dependent glutaminase, GDH (GLUD1) glutamate dehydrogenase, PEPCK (PCK1) phosphoenolpyruvate carboxykinase
Kidneys also display a bicarbonate-secreting mechanism in the collecting duct. Pendrin (SLC26A4), a Cl−/HCO3− exchanger functioning as a bicarbonate secreting protein, was also identified in type B and in non-A non-B intercalated cells [108]. The basic mechanism is the same here, a proton moves through the basolateral membrane and a bicarbonate is secreted to the apical lumen. It has been suggested that pendrin is a key factor in the renal defense against alkalosis given that isolated cortical collecting ducts from alkali-loaded pendrin null mice cannot properly secrete bicarbonate [99]. In addition, these mice are prone to develop alkalosis under dietary sodium and potassium restriction [92]. The gastrointestinal hormone secretin stimulates cystic fibrosis transmembrane conductance regulator (CFTR) and pendrin activity. They work in concert promoting bicarbonate secretion in type B intercalated cells in the collecting duct [7]. Patients with cystic fibrosis carrying a mutation in CFTR show impaired renal excretion of bicarbonate [6]. Authors suggested that secretin would be a bicarbonate-regulating hormone and would be responsible for the elevated bicarbonate excretion after a meal, which is known as alkaline tide [7]. In addition, a role for pendrin in salt regulation has been proposed, and acid–base changes could also be secondary to changes in extracellular volume [122, 124]. Besides CFTR, pendrin may also function in concert with NDCBE1 (SLC4A8), a Na+/HCO3−/Cl− transporter. Pendrin and NDCBE1 would generate net reabsorption of NaCl while limiting bicarbonate secretion [124].
The second fundamental activity is the formation of de novo or new bicarbonate, which means the restoration of bicarbonate consumed by the addition of fixed acids to the organism. This new bicarbonate is formed via two main mechanisms, ammoniagenesis and excretion of titratable acids. These mechanisms were identified in the first decades of the XX century, when Henderson recognized that excretion of ammonium and phosphates were essential for the maintenance of acid–base balance [51, 52]. Other forms of urinary acids were already recognized at that time [40], and these acids were termed titratable acids (but also received other names, such as free acids) [105, 121].
Titratable acid excretion is a simple process which is a consequence of a proton secreted binding bases other than bicarbonate. Therefore, bicarbonate is formed inside the cell without consumption of bicarbonate in the lumen. On the other hand, bicarbonate formation through ammoniagenesis requires steps that span almost the whole nephron. The formation of ammonium happens in the proximal tubule via biochemical reactions that start from glutamine. This amino acid is metabolized in the mitochondria producing alpha-ketoglutarate, which participates in the cytosolic gluconeogenesis. Mitochondrial and cytosolic steps yield together a net of two molecules of bicarbonate and ammonia per glutamine. Ammonia (NH3) is secreted to the tubular lumen and with H+ forms ammonium (NH4+) (Fig. 1). If the nephron ended after the proximal tubule, this would be the end of this story with positive formation of bicarbonate (new bicarbonate). However, if ammonium goes back to the bloodstream, it will consume bicarbonate in the liver via the urea cycle, which will deny new bicarbonate formation. Ammonium excretion does not follow the path of many other ions that travel through the tubule lumen to the ureter, but most of it is reabsorbed in the thick ascending limb and secreted back into the thin descending limb of the loop of Henle or in the collecting duct, thus partly bypassing the distal convoluted tubule. Different hypotheses have been proposed to explain why ammonium would take such an unconventional path before reaching the urine. As mentioned previously, this countercurrent multiplication of ammonium could avoid its reabsorption in the cortex [88, 112] (i.e., distal convoluted tubule and cortical collecting duct), but it was also suggested that NH4+ would contribute to NKCC2 activity and NaCl reabsorption in the thick ascending limb [128, 129]. Regardless of the potential reasons why these mechanisms could have been fixed, ammonium crosses the apical membrane of the thick ascending limb via NKCC2 substituting potassium in the process. It leaves the cell through the basolateral membrane via two mechanisms, NHE4 (NH4+ instead of H+ exchanged with Na+) or the coordinated transport of NH3 to the medullary interstitium in parallel with the movement of HCO3− into the cell involving the electroneutral Na+-bicarbonate cotransporter NBCn1 [16, 87].
In the medullary interstitium, sulfatides facilitate the retention of ammonium, which passively diffuses as ammonia into the collecting duct via the RhCG and maybe also as ammonia or ammonium via RhBG [12, 23, 113]. Proton secretion by type A intercalated cells in parallel with ammonia transport traps ammonium into the lumen, increasing the probability of its excretion in the urine. Only after this journey is the production of new bicarbonate consolidated. Despite the observation that excretion of titratable acids and ammonia were essential for the maintenance of acid–base balance in the first years of the XX century, only in 1921 did Nash and Benedict demonstrate that ammonia was formed in the kidneys [83]. Subsequent decades saw a stream of studies trying to identify the metabolic origins of ammonium and how this mechanism was regulated in health and disease.
Current views on the pathophysiology of metabolic acidosis in kidney disease
In this section, we cover common conditions of renal origin that generate metabolic acidosis and its management in the clinical setting.
Metabolic acidosis in CKD
In CKD, metabolic acidosis occurs with declining kidney function and the subsequent fall of glomerular filtration rate (GFR) independent of the underlying kidney disease. The consequent loss of nephrons results in two important processes: globally reduced excretion of ammonia, but increased ammoniagenesis in the remaining nephrons. In addition, hemoglobin also functions as a buffer in the blood, and CKD is commonly accompanied by anemia, which might contribute to metabolic acidosis. Moreover, a series of phosphaturic mechanisms preserve titratable acid excretion in patients with CKD, but in end-stage kidney disease, reduction in its excretion contributes to the occurrence of overt metabolic acidosis [81]. Clinically, metabolic acidosis presents not only as normal anion gap metabolic acidosis but, in some patients, and especially in advanced stages, also as anion gap metabolic acidosis. However, a more recent theoretical construct includes earlier CKD stages by using the term “eubicarbonatemic metabolic acidosis,” which is defined by proton accumulation preceding the fall of serum bicarbonate levels [44]. Noteworthy, dietary acid load has a key role in determining the occurrence of eubicarbonatemic or overt metabolic acidosis in individuals with compromised kidney function [131].
Kidneys respond to metabolic acidosis by stimulating mechanisms that form new bicarbonate. Given that pH has highly pleiotropic effects, it is wise to look at the adaptive responses to metabolic acidosis and what possible effects their chronic activation could cause. Along these lines, Nath et al. proposed that accumulation of ammonium in the renal interstitium would trigger inflammation via the alternative complement pathway [85]. They identified that adding NaHCO3 to the diet reduced ammonium concentration in the renal vein and attenuated intratubular casts, tubular dilation, and interstitial fibrosis. One of the hallmarks of chronic kidney disease is the reduction in ammonium excretion. However, Simpson showed in 10 patients with CKD and acidosis that GFR falls proportionally more than ammonium excretion, which suggests that ammonium generation per nephron may increase in acidotic patients with CKD [104]. However, it has been demonstrated that ammonium binds sulfatides in the medullary interstitium [113]. Therefore, it is not clear how ammonium could trigger the activation of the complement system, unless intrarenal sulfatides are also reduced in CKD. Some key open questions need to be addressed in relation to the NH4+/alternative complement system hypothesis: (1) Are all forms of CKD marked by accumulation of NH4+ in the renal tissue? (2) Is this mechanism relevant both in the cortex and in the medulla? (3) Is NH4+ the actual molecule responsible for triggering inflammatory responses in CKD with acidosis? (4) What other immune responses beyond activation of the alternative complement pathway could be triggered by NH4+?
Kidneys also increase the activity of NHE3 in the proximal tubule in response to acidosis, which is assumed to be a mechanism supporting ammonium excretion via a Na+/NH4+ exchange [32]. At the same time, proton secretion in the collecting duct is increased which helps ammonia to be converted into ammonium and then be trapped into the tubular lumen. The hormones angiotensin II, aldosterone, and endothelin-1 support the increase in these mechanisms in the proximal tubule and collecting duct [135]. However, their chronic activation by acidosis leads to inflammatory processes and fibrosis. Studies in nephrectomized rats and patients with CKD support this hypothesis [132,133,134]. However, a recent randomized clinical trial with 45 patients with CKD designed to identify potential benefits of alkali therapy on the reduction of these hormones did not find a reduction in the levels of urinary renin, angiotensinogen, aldosterone, or endothelin-1 [17]. While urinary levels of these hormones might not reflect their intrarenal levels, further studies are necessary to evaluate the effectiveness of alkali therapy in reducing these harmful factors in CKD. In summary, activation of the alternative complement pathway by ammonium (published in 1985) and the toxic effect of hormones stimulated by acidosis (as shown in a long list of studies led by Donald Wesson and Jan Simoni and supported by many colleagues since the 1990s) have been established as the modern explanations for the deleterious effects of acidosis on CKD. They link the physiological responses of the kidneys against metabolic acidosis to inflammatory processes.
Renal tubular acidosis
Renal tubular acidosis (RTA) is a condition in which tubular secretion of H+ and reabsorption of HCO3− are impaired despite relatively normal GFR [110]. It was first described in the 1930s in pediatric patients with severe renal calcification, but only in the next decade would the condition be explained [2, 22, 71]. It was termed “renal acidosis,” a condition of “tubular insufficiency without glomerular insufficiency” [2]. Renal tubular acidosis is clinically characterized by normal anion gap metabolic acidosis with an alkaline urinary pH. Particularly in the last decades, deeper insights have been gained on genes involved in inherited forms of renal tubular acidosis. Dependent on the gene and localization of the defect, three different types of renal tubular acidosis are defined: (1) proximal RTA, (2) distal RTA, and (3) hyperkalemic RTA. By now, more than 25 genes have been identified to cause inherited RTA. Interestingly, polymorphisms may also cause some diseases that do not present typically as inherited RTA in patients with nephrocalcinosis or nephrolithiasis [123]. Moreover, there are still patients with inherited RTA where no mutation has been found yet, indicating that other genes or further mutations, for example, in noncoding regions, may be involved.
Hyperkalemia is commonly accompanied by lower net acid excretion and metabolic acidosis [36]. A recent study in mice has added some evidence on the role of hyperkalemia per se in the pathogenesis of hyperkalemic RTA [48]. Hyperkalemia causes metabolic acidosis by both reducing ammoniagenesis in the proximal tubule and impairing ammonia transport in the collecting duct [48]. Interestingly, deletion of Kir4.2 (Kcnj15) in mice disturbs the membrane potential of the proximal tubule basolateral membrane, which elevates intracellular pH and reduces ammoniagenesis, causing proximal RTA [11]. Regarding acquired forms of RTA, few studies have shed light on a specific autoimmune disease called Sjögren syndrome [30, 125]. This is a systemic disease that can involve the kidney by defective urinary acidification and subsequent distal RTA. Published data suggest that autoantibodies may be involved in the pathogenesis by potentially affecting acid-secreting type A intercalated cells in the distal tubule [120]. However, more studies are required to identify the respective antigens that may be targeted by the autoantibodies.
Metabolic acidosis in kidney transplant recipients
Interestingly, metabolic acidosis occurs in kidney transplant recipients (KTRs) at higher eGFR levels when compared to patients with CKD [79]. This finding suggests that there may be transplant-specific mechanisms involved, and it is further supported by the fact that metabolic acidosis in KTRs typically presents with the features of renal tubular acidosis (RTA), such as normal anion gap metabolic acidosis, compared to high anion gap acidosis in patients with CKD [79]. Among the transplant-specific features, calcineurin inhibitors may be of great importance. Data from animal and human studies demonstrated that both cyclosporine and tacrolimus may affect tubular function including recent findings about the role of pendrin in the pathogenesis of distal RTA [4, 50, 74, 80, 127]. In addition, other elements, such as immunological factor associated with allograft rejection, donor-associated factors (graft from a deceased donor may be associated with a higher rate of metabolic acidosis) [18, 89], and dietary factors (white meat is associated with lower risk and dietary acid load is associated with a higher risk of graft failure) [100, 140], may also contribute to the development of metabolic acidosis in KTRs. In a recent study, we investigated the impact of metabolic acidosis and its therapy on molecular changes in renal biopsies of KTRs via RNA sequencing and immunofluorescence [59]. Our data demonstrated that metabolic acidosis in kidney transplant recipients is associated with changes in the renal transcriptome and protein expression of genes mostly involved in acid–base transport and cell energy metabolism (see section on “Metabolic acidosis and metabolism”). These changes were partly reconstituted by alkali therapy [59].
Impact of alkali therapy on kidney function
Metabolic acidosis is common in patients with CKD, with an increasing prevalence in advanced stages of CKD. Starting in 2009, with the first open-label clinical trial, more than 10 studies have investigated the impact of alkali therapy on kidney function and CKD progression as well as proteinuria [55]. Although when comparing alkali therapy with placebo or no medication, the results were favoring sodium bicarbonate to slow CKD progression, the overall certainty of evidence is still low, and further studies are required.
Alkali therapy in transplantation
Similar to CKD, metabolic acidosis is highly prevalent after kidney transplantation, with a reported prevalence of 12 to 58% [137]. Although by now many interventional trials have evidenced the beneficial effect of alkali therapy on preservation of kidney function in patients with CKD, no prospective randomized controlled trial has been published yet on the potential impact of alkali supplementation on graft function in kidney transplant recipients. However, a few retrospective analyses indicate an association of metabolic acidosis and graft survival [18, 89]. The first study was an observational multicenter analysis of 2318 Korean KTRs with metabolic acidosis (defined as tCO2 level < 22 mmol/l), demonstrating an association of metabolic acidosis with graft outcome and mortality [89]. A more recent study from France including 914 KTRs confirmed these data and reported low bicarbonate being predictive for allograft loss [18]. Furthermore, two observational studies from our center showed a positive correlation of serum bicarbonate with eGFR in the first year after transplantation and a significant association of serum bicarbonate with long-term graft and patient survival [136, 138]. A retrospective study from Japan with non-KTR patients with CKD concluded that venous pH altered the association between CKD and progression to kidney replacement therapy [62]. In other words, patients with low venous bicarbonate and acidemia were under higher risk of undergoing kidney replacement therapy than patients with low venous bicarbonate and normal blood pH. It is unknown whether the same modulation occurs between serum bicarbonate or tCO2 and graft failure/loss.
The Preserve Transplant Study is an investigator-initiated prospective randomized placebo-controlled single-blinded interventional trial investigating the effect of alkali treatment on graft function of KTRs with metabolic acidosis (defined as serum bicarbonate ≤ 22 mmol/l) over 2 years. Kidney graft recipients, ≥ 18 years of age, at least 12 months after transplantation, with an eGFR between 15 and 89 ml/min/1.73 m2 and bicarbonate of ≤ 22 mmol/l were randomized 1:1 to receive placebo or sodium hydrogen carbonate. The results of the study are expected in 2022 and will be essential to clarify whether alkali treatment in KTRs with metabolic acidosis may help to prolong long-term graft survival in this population.
Metabolic acidosis and metabolism
Claude Bernard observed that dietary habits from carnivores and herbivores would determine the acidity or alkalinity of the urine, therefore recognizing that what we eat imposes different challenges to the organism from an acid–base perspective [8]. The incomplete oxidation of substrates was already recognized as a source of acidification of the organism in the 1898s Der Diabetes Mellitus, by Bernhard Naunyn, who termed this condition as Acidose (acidosis) [9]. Since then and for several decades, substantial investigation on acid–base disorders focused on whole-organism metabolic studies in an attempt to identify how metabolites in humans and animal models like rabbits and dogs would respond to alkaline and acid challenges. After two decades of debate on the source of renal ammonia, glutamine was identified by Donald Van Slyke et al. in 1943 as the main fuel of ammoniagenesis in response to metabolic acidosis, a process further understood in the subsequent decades [84, 94, 106]. The close relationship between renal gluconeogenesis and ammoniagenesis (and new bicarbonate formation) ties cell energy metabolism to acid–base balance even further. Glutamine, lactate, and glycerol are the main substrates of renal gluconeogenesis, but glycerol and lactate do not participate in the stimulated ammoniagenesis in response to low pH (although lactate oxidation also yields one HCO3−) [95, 115]. During acidosis, there is a shift from the metabolization of other substrates of gluconeogenesis (e.g., lactate) towards glutamine [93]. Moreover, substrates of the TCA cycle inhibit ammoniagenesis in normal acid–base conditions, but have a less inhibitory effect during acidosis, while glycerol has almost no effects on ammoniagenesis [3]. However, patients with CKD with mildly impaired kidney function and partially preserved ammonium excretion showed almost negligible renal extraction of glutamine [119]. Another study with patients with CKD receiving oral intake of glutamine demonstrated that ammonium excretion could not be increased even under abundant availability of glutamine [130]. Authors suggested that a reduction in the enzymatic activities participating in ammoniagenesis would explain the reduced ammonium excretion. Indeed, it was later demonstrated that acute injury to the kidneys reduces the expression of ammoniagenic enzymes [68] and that both in acute kidney injury and chronic kidney disease, there is a metabolic rewiring that redirects energy metabolism away from gluconeogenesis [39]. In healthy humans and animal models, stimulation of ammoniagenesis and gluconeogenesis by acidosis increases renal glucose generation [1, 111]. Therefore, pH affects biochemical pathways that will lead to differential production and release of metabolites. The focus on cell metabolism as a key element to understand kidney disease has increased in the past recent years. Integrative approaches combining molecular and clinical data have found that metabolism and inflammation are central pathways in various forms of CKD [37, 63, 77]. Interestingly, when we subjected mice to a crystal nephropathy CKD model and treated them with oral bicarbonate, two largely restored pathways were again inflammation and metabolism [90]. Moreover, as mentioned earlier, we collected renal biopsies of kidney transplant recipients both with and without acidosis and with comparable eGFR and performed RNA sequencing. More than half of the altered genes between acidotic and non-acidotic patients were enzymes, mostly involved in cell energy metabolism activities like beta oxidation, fatty acid synthesis, interconversion between L-methionine and L-homocysteine, and others [59] (Fig. 2). We also obtained a few biopsies of patients with acidosis and treated with alkali therapy and showed restoration of genes involved in bicarbonate transport (NBCe1, pendrin, and Kir4.2), beta oxidation (ACADSB), and interconversion of L-homocysteine and L-methionine as well as glycine and L-serine (SHMT1) (Fig. 2).
Summary of main renal metabolic pathways altered between kidney transplant recipients (KTRs) with or without acidosis. Bulk RNA sequencing data using RNA from kidney biopsies of KTRs identified genes altered between patients with or without acidosis, but with comparable eGFR. These genes participate in metabolic activities shown in this figure in black. Red lines show molecular pathways that had genes restored by alkali therapy. Blue arrows show direct biochemical reactions, and blue dashed lines show indirect biochemical reactions. Black arrows show movement of molecules. Data originally published in [59]. TCA cycle tricarboxylic acid cycle (also citric acid cycle or Krebs cycle), P5P pyridoxal-5′-phosphate, GSH glutathione, THF tetrahydrofolate
Metabolic acidosis alters the redox state of mitochondrial nicotinamide adenine dinucleotide (NAD) and causes mitochondrial stress in renal proximal tubules, which affects lipid metabolism [21, 82]. Bugarski et al. have shown that oxidation of NAD by acid load injures proximal tubule cells and that alkali treatment prevents such changes [21]. Low-grade metabolic acidosis is also a necessary signal for mitochondrial remodeling in response to hypoxia. Cortical neurons exposed to pH 6.5 showed increased crista number and sustained functional efficiency under hypoxic conditions while mitochondrial fragmentation and cell death were prevented [64]. However, exposure of the same cells to more alkaline pH (6.8–7.2) or more acidic (pH 6.0) induced mitochondrial fragmentation. Renal cells are exposed to hypoxic conditions in chronic kidney disease, and similar mechanisms may operate. Also in neurons, ASIC1a mediates pH-dependent calcium transport into the mitochondria, increasing respiration and metabolic rate [101]. Accordingly, whole kidney mitochondria from rats exposed to 48 h of 0.25 M NH4Cl in the drinking water showed faster calcium uptake and higher resting respiration [5]. On the other hand, lipid accumulation in opossum kidney cells (OKP) cells, a model of renal proximal tubule cells, inhibits ammonium secretion, a process that is similarly observed in Zucker diabetic fatty (ZDF) rats, a model of type 2 diabetes [13, 14]. Therefore, acid–base status directly influences proximal tubular glutamine metabolism with impact on gluconeogenesis and lipid metabolism, while impaired lipid metabolism or gluconeogenesis impacts renal capacity of excreting acids.
Two central questions derive from these observations: (1) Does deranged metabolism define trajectories towards faster or slower kidney function decline in chronic kidney disease (or recovery vs. declining kidney function in an AKI to CKD scenario) or does it simply reflect disturbance from other causes? Cippà et al. identified early markers associated with these trajectories in biopsies of patients submitted to renal ischemia and reperfusion because of transplantation [29]. They identified that genes associated with mitochondrial function, senescence, and inflammation were among the most prevalent genes associated with different trajectories. (2) Given the roles of pH in metabolism and mitochondrial function described previously here, is pH a key factor influencing these trajectories, or is it only a sensitive biomarker of kidney damage?
Cell energy metabolism beyond the proximal tubule
It is evident from the discussion above that our knowledge in renal energy metabolism is highly centered on the proximal tubule metabolism. However, other segments are also essential for the acid–base balance, and further research is necessary to shed light on the role of cell metabolism in the whole nephron in health and disease. Intercalated cells are an intriguing case for the study of renal cell energy metabolism. While animal cells are energized by Na+/K+ ATPase activity, there are strong evidences that intercalated cells do not express this protein and are rather energized by a H+-ATPase [25]. Intercalated cells display a sizeable Golgi apparatus and are also known as mitochondria-rich cells, a term that comes from the higher proportion of mitochondria in comparison to principal cells [28, 60]. Type A intercalated cells accumulate mitochondria in the apical cell pole and do not present the typical enrichment of mitochondria next to the basolateral membrane [28, 61]. Renal epithelial cells in the cortex rely mostly on oxidative phosphorylation to generate ATP, but type A intercalated cells have a high anaerobic glycolytic capacity, which may produce the driving force for H+ secretion [10, 42, 116]. Urinary acidification capacity is mostly preserved in CKD [75, 102], but it is compromised in renal tubular acidosis. Whether tubulointerstitial injury and distal RTA impose or are associated with differential metabolic demands on type A intercalated cells is still an open question. Given the potential role of the thick ascending limb in urine acidification [33], these studies should also cover the loop of Henle.
How would alkali therapy protect renal metabolism?
Metabolic acidosis in CKD is a consequence of nephron loss and reduced ammoniagenesis. With less functional units, kidneys lose capacity for generating new bicarbonate and slowly lose the battle against daily metabolic acidification. Given that the remaining functional nephrons must deal with multiple tasks other than acid–base balance, we hypothesize that kidneys may need to sacrifice efficiency in certain functions to keep homeostasis of multiple parameters at acceptable levels. There are hints supporting this hypothesis. NRF2 is a ubiquitously expressed master regulator of oxidative stress, with roles in intermediary metabolism and mitochondrial function [49, 65]. Mouse deficient for NRF2 (Nfe2l2) have strongly downregulated expression of SNAT3, the main importer of glutamine in the proximal tubule and a crucial player in the response to metabolic acidosis and ammoniagenesis [72]. To our surprise, acid-loaded Nfe2l2 knockout mice showed the same severity of metabolic acidosis in comparison with wild-type mice under the same conditions. However, after a week under acid-loaded conditions, Nfe2l2-deficient mice show elevated markers of kidney injury and oxidative stress despite a similar grade of acidosis in relation to wild-type mice [72]. Potentially, with cells trying to cover too many tasks at the same time, some slowly fall behind. Alleviating acid–base stress could therefore be a way of releasing the pressure on one of the multiple tasks that a cell has to handle in pathological conditions. Parallel scenarios can be also observed in other contexts. For example, metabolomic analysis has shown that acidosis induces cellular metabolism reprogramming of solid tumors via NRF2 [67]. Authors observed that intermediate metabolites are redirected away from other important metabolic processes in solid tumors during acidosis. However, additional investigation is necessary to demonstrate that acidosis exacerbates metabolic stress in kidney disease.
Early markers of eubicarbonatemic metabolic acidosis
Chronic kidney disease leading to eubicarbonatemic acidosis is a plausible hypothesis for at least two reasons. First, why would kidneys undergoing reduced kidney function suddenly fail to control acid–base balance if not in a slow and undetectable fashion until systemic markers change beyond the range of normality? Second, acid–base disorders are primarily determined by blood gas analysis, which is an exam using blood as material for investigation. Blood is not an isolated solution in a hermetically confined pipe, but a solution in continuous exchange with the interstitial space and then with the cells. Where would acidosis start? This depends on the source of acidosis. If it occurs via CO2 intoxication or ingestion of acid, we would have an extracellular-intracellular cause. But in the case of kidney disease, if fixed acids originated from the metabolism are the acidifying factor and kidneys are not capable of adjusting to this daily challenge, the origin of the acidosis comes from the cells and therefore occurs in an intracellular-extracellular fashion (i.e., from the net endogenous acid production). Acids generated in the intracellular space would not only meet intracellular buffers but would also move to the interstitium meeting the next layer of buffers. The effect in the bloodstream could only be visible after several layers of protection fail. Therefore, the closer we look at the origin of the event, the earlier we could detect the derangement (a counter-argument against this potentially reductionist approach would be the loss of information because of emergent properties, but it does not seem plausible in this case given that this is just a shift in focus within the same level and scale [86]). Urine citrate excretion has been proposed as an early marker of acid retention [45]. Citrate is a key factor in cell energy metabolism participating in the citric acid cycle or Krebs cycle. The metabolization of citrate yields a net gain of bicarbonate. The principle is that an organism undergoing acid retention would reabsorb more citrate, and less citrate would appear in the urine. The strategy of measuring urine citrate goes along with what is proposed here: that cell energy metabolism is essential to understand acid–base disorders and that the closer one surveys the origin of the event, the earlier it could be detected. The problem is that citrate is not only affected by acid–base conditions, but also by multiple other metabolic requirements of the cell. Further demonstration that it could be a useful marker has been published [43]. These observations must be expanded, and a panel of metabolites representing accurately the acid–base condition of the cell might substitute this or other single markers in the future.
Additional mechanisms
A series of additional mechanisms has been proposed and most probably plays relevant roles in the outcomes of acute kidney injury, acute kidney disease, and chronic kidney disease. We briefly explore some of these mechanisms below.
Klotho
Klotho functions as a co-receptor of fibroblast growth factor 23 (FGF23) and mediates phosphate excretion, the main titratable acid. Additionally, it has renoprotective effects and regulates inflammation [54]. It exists both as a membrane-bound or soluble molecule. High pH activates the calcium-sensing receptor (CaSR) in the distal convoluted tubule which activates a disintegrin named metalloproteinase 10 (ADAM10) [141]. The disintegrin cleaves membrane-bound klotho, generating soluble α-klotho. Low pH has opposite effects. Patients with CKD showed reduced α-klotho levels early in the disease, and alkali therapy increased excretion of α-klotho in a pilot study with patients with CKD [46]. Therefore, it is tempting to speculate that alkali therapy protects kidney function also via protection of klotho levels. Mice subjected to a crystal nephropathy model and treated with alkali therapy show preserved renal α-klotho levels despite severe tubulointerstitial injury [90].
pH sensing
The proper response of the kidneys and lungs to acid–base challenges relies on precise pH sensing by the kidneys and peripheral and central chemoreceptors. Kidneys express a large array of proteins in which H+ functions as an allosteric modulator or a ligand. They are ionic channels, enzymes, and G protein-coupled receptors (GPCRs). Some examples are TWIK-related acid-sensitive K + channel (TASK) [20], acid-sensing ion channels (ASICs) [24], insulin receptor–related receptor (IRRR) [34], soluble adenylyl cyclase (sAC) [98], ovarian cancer G protein-coupled receptor 1 (OGR1/Gpr68), G protein-coupled receptor 4 (GPR4), and T cell death-associated gene 8 (TDAG8/Gpr65) [57]. Moreover, an intracellular pH-senstive proline-rich tyrosine kinase 2 (PYK2) and a bicarbonate/CO2 sensing protein receptor protein tyrosine phosphatase (RPTPgamma) have also been identified [15, 69]. Disruption of several of these sensing mechanisms leads to poor management of acid–base balance by the kidneys or to insensitivity of mechanisms regulated by acid–base conditions. For example, TASK2 knockout mice display a phenotype similar to human proximal renal tubular acidosis [126], and OGR1 knockout mice display poor coordination between urinary acidification and calcium excretion [58]. GPR4 deficiency in mice fully blunts acid-dependent proliferation of type A intercalated cells and induction of transporters involved in acid–base balance in these cells [27]. These animals also show lower excretion of titratable acids and lower ammonium excretion in response to acid load [117, 118]. However, this might be indirectly caused by the respiratory acidosis of central origin observed in these animals [27, 66]. The role of pH sensing is also extended to kidney injury conditions, as seen in the inhibition of ASIC1a with psalmotoxin 1 (PcTx1) in mice, which attenuated injury caused by renal ischemia reperfusion [109]. PcTx1 not only increases the affinity of ASIC1a by H+, but also functions as an agonist of ASIC1b in neurons [26]. GPR4-deficient mice are also protected from renal ischemia reperfusion injury [35]. In summary, pH sensing mechanisms play central roles in acid–base balance in health and in pathological processes. However, despite extensive research on either the pH-sensing properties of these proteins or on their role in disease, there are only few studies that reported a systematic investigation of how both aspects interact. There is plenty of room for research in this direction.
Acid–base and immune responses
The interaction between acid–base conditions and immune responses has been explored by research in several fields, such as oncology, pain and nociception, pulmonology, and gastroenterology, but it has been understudied by renal physiologists and nephrologists [31, 56, 57, 91, 143]. Acid–base conditions influence differentiation and motility of immune cells and their capacity to release substances [38, 97, 142]. Given the increasing attention towards the role of inflammation in kidney diseases, it is expected that this gap will be narrowed in the next few years. In the previous sections, we explored some examples of this interaction, such as ammonium as a trigger of the alternative complement pathway and acidosis-stimulated chronic activation of hormones driving inflammation of the renal tissue. The effect of acidosis in the immune cell activity in kidney disease is mostly unknown and deserves special attention. Recently, we showed that CD4+ T cells and inflammatory monocyte levels were reduced in kidneys of mice subjected to a crystal nephropthy model and receiving oral bicarbonate [90]. However, alkali therapy could also have extrarenal effects with relevance to the kidneys. A hypertensive kidney disease rat model (Dahl salt-sensitive) under oral bicarbonate intake displayed splenic and renal macrophage polarization towards an anti-inflammatory M2 type suggesting that alkalinization impacts pre-renal differentiation of immune cells with impact in the kidneys [96]. Interestingly, administration of esomeprazole, a proton pump inhibitor, blunted the anti-inflammatory effect of NaHCO3 intake in rats. Authors proposed a mechanism that gastric acidification is directly or indirectly sensed by mesothelial cells of the peritoneum which sends an anti-inflammatory message via cholinergic signals to the spleen [96]. This novel mechanism would at least partially bypass canonical acid–base sensing systems like peripheral and central chemoreceptors. However, its actual impact in kidney function has yet to be demonstrated. Oral bicarbonate could also influence kidney disease through the relationship between intestinal dysbiosis and mitochondrial dysfunction in CKD [76]. Concerns with ocean acidification have prompted multiple studies aimed at identifying whether lower pH could impact the diversity of microorganisms in the sea. Hypercapnia affected the intestinal microbiota of fish and crab [41, 73]. Likewise, there is a clear impact of ruminal acidification on the microbiota of different species of cattle [53, 70]. Acidic water has been shown to affect intestinal microbiota of mice [107, 139], but results are contradictory and have not been reproduced by others [144]. Moreover, the single study performed in humans did not find any impact of acidic water in the intestinal microbiota of young males [47].
Expanded conceptual framework integrating interactions between pH homeostasis and progression of chronic kidney disease
Most probably, certain effects of pH on kidney function act independently of each other. However, independent effectors may synergize into further kidney injury, and some others may actually be dependent on each other. Here, we propose a conceptual framework with multiple known factors involved in acid–base-dependent progression of chronic kidney disease and how they would evolve from early to late stages of CKD (Fig. 3). In this figure, we also list key open questions related to multiple steps of this framework.
Conceptual framework how chronic kidney disease, inflammation, and deranged metabolism form a vicious cycle involving metabolic acidosis as an engine. Nephron loss and impaired renal function reduce kidney capacity of eliminating acids and generating new bicarbonate which leads to accumulation of acids in the organism. Renal responses to acidosis exacerbate inflammation and deranged metabolism that ultimately reduce kidney function and kidney capacity of keeping pH homeostasis. Steps of this network are shown in continuous black boxes, and open questions related to each of these steps are shown next to it in dashed black boxes. Inflammation and metabolism domains are artificially delimited in different colors as some of these steps may belong to both domains
Concluding remarks
Extensive research in the XX century characterized how metabolic activities of all cells generate the input of the acid–base balance and how mainly the kidneys and lungs control the output. Dietary habits influence net production of acids and bases and are important factors determining the daily stress to pH homeostasis. Recent research has identified that metabolism and inflammation are central pathways to multiple forms of kidney disease, and there is evidence that acid–base status is a potent modulator of these pathways. Models proposed in the past couple of decades on how acidosis impairs kidney function in chronic kidney disease and how alkali therapy protects kidney function did not include the role of cell metabolism (and potentially immunometabolism) as core pieces. H+ has pleiotropic effects in biological systems, and it is believed to affect organisms from multiple angles. It is time to revisit relevant knowledge acquired since the late XIX century and further elaborate a holistic framework that includes this diversity. However, certain factors might have a more dominant effect than others, and identifying them may be a powerful tool to manipulate pathways involved in the progression of chronic kidney disease. We propose that cell metabolism is at the core of the pH-dependent events associated with the progression of chronic kidney disease. If this is the case, a deep understanding of how pH affects biochemical reactions in a systemic fashion may provide a powerful tool to control the progression of renal and extrarenal chronic diseases with deranged metabolism.
Change history
23 May 2022
Update of the author name tagging for the correct author name citation.
References
Aber GM, Morris LO, Housley E (1966) Gluconeogenesis by the human kidney. Nature 212:1589–1590. https://doi.org/10.1038/2121589a0
Albright F, Burnett CH, Parson W, Reifenstein ECJ, Roos A (1946) Osteomalacia and late rickets; the various etiologies met in the United States with emphasis on that resulting from a specific form of renal acidosis, the therapeutic indications for each etiological sub-group, and the relationship between osteomalacia and Milkman’s syndrome. Medicine 25:399–479
Bagnasco SM, Gaydos DS, Risquez A, Preuss HG (1983) The regulation of renal ammoniagenesis in the rat by extracellular factors. III. Effects of various fuels on in vitro ammoniagenesis. Metabolism 32:900–905. https://doi.org/10.1016/0026-0495(83)90204-4
Banki E, Fisi V, Moser S, Wengi A, Carrel M, Loffing-Cueni D, Penton D, Kratschmar DV, Rizzo L, Lienkamp S, Odermatt A, Rinschen MM, Loffing J (2021) Specific disruption of calcineurin-signaling in the distal convoluted tubule impacts the transcriptome and proteome, and causes hypomagnesemia and metabolic acidosis. Kidney Int 100:850–869. https://doi.org/10.1016/j.kint.2021.06.030
Bento LMA, Fagian MM, Vercesi AE, Gontijo JAR (2007) Effects of NH4Cl-induced systemic metabolic acidosis on kidney mitochondrial coupling and calcium transport in rats. Nephrol Dial Transplant 22:2817–2823. https://doi.org/10.1093/ndt/gfm306
Berg P, Svendsen SL, Sorensen MV, Larsen CK, Andersen JF, Jensen-Fangel S, Jeppesen M, Schreiber R, Cabrita I, Kunzelmann K, Leipziger J (2020) Impaired renal HCO3—excretion in cystic fibrosis. J Am Soc Nephrol 31:1711–1727. https://doi.org/10.1681/ASN.2020010053
Berg P, Svendsen SL, Sorensen MV, Schreiber R, Kunzelmann K, Leipziger J (2021) The molecular mechanism of CFTR- and secretin-dependent renal bicarbonate excretion. J Physiol 599:3003–3011. https://doi.org/10.1113/JP281285
Bernard C (1859) Leçons sur les propriétés physiologiques et les altérations pathologiques des liquides de l’organisme. Bailleère, Paris
Naunyn Bernhard 1839–1925, Royal College of Physicians of Edinburgh (1898) Der Diabetes melitus. Wien : Alfred Hölder
Bhargava P, Schnellmann RG (2017) Mitochondrial energetics in the kidney. Nat Rev Nephrol 13:629–646. https://doi.org/10.1038/nrneph.2017.107
Bignon Y, Pinelli L, Frachon N, Lahuna O, Figueres L, Houillier P, Lourdel S, Teulon J, Paulais M (2020) Defective bicarbonate reabsorption in Kir4.2 potassium channel deficient mice impairs acid-base balance and ammonia excretion. Kidney Int 97:304–315. https://doi.org/10.1016/j.kint.2019.09.028
Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM (2008) A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456:339–343. https://doi.org/10.1038/nature07518
Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW (2008) Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol 294:F1315–F1322. https://doi.org/10.1152/ajprenal.00550.2007
Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW (2009) Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol 297:F1419–F1426. https://doi.org/10.1152/ajprenal.00177.2009
Boedtkjer E, Hansen KB, Boedtkjer DM, Aalkjaer C, Boron WF (2016) Extracellular ʜ c o3 is sensed by mouse cerebral arteries: regulation of tone by receptor protein tyrosine phosphatase γ. J Cereb Blood Flow Metab 36:965–980. https://doi.org/10.1177/0271678X15610787
Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR, Houillier P (2010) NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest 120:1895–1904. https://doi.org/10.1172/JCI36581
Bovée DM, Roksnoer LCW, van Kooten C, Rotmans JI, Vogt L, de Borst MH, Zietse R, Danser AHJ, Hoorn EJ (2020) Effect of sodium bicarbonate supplementation on the renin-angiotensin system in patients with chronic kidney disease and acidosis: a randomized clinical trial. J Nephrol. https://doi.org/10.1007/s40620-020-00944-5
Brazier F, Jouffroy J, Martinez F, Nguyen-Khoa T, Anglicheau D, Legendre C, Neuraz A, Prié D, Bienaimé F (2020) Association of blood bicarbonate and pH with mineral metabolism disturbance and outcome after kidney transplantation. Am J Transplant 20:1063–1075. https://doi.org/10.1111/ajt.15686
Brosius F, Nguyen K, Stuart-Tilley A, Haller C, Briggs J, Alper S (1995) Regional and segmental localization of AE2 anion exchanger mRNA and protein in rat kidney. Am J Physiol 269:F461–F468
Buckler KJ (2015) TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch - Eur J Physiol 467:1013. https://doi.org/10.1007/s00424-015-1689-1
Bugarski M, Ghazi S, Polesel M, Martins JR, Hall AM (2021) Changes in NAD and lipid metabolism drive acidosis-induced acute kidney injury. J Am Soc Nephrol 32:342–356. https://doi.org/10.1681/ASN.2020071003
Butler AM, Wilson JL, Farber S (1936) Dehydration and acidosis with calcification at renal tubules. J Pediatr 8:489–499. https://doi.org/10.1016/S0022-3476(36)80111-5
Caner T, Abdulnour-Nakhoul S, Brown K, Islam MT, Hamm LL, Nakhoul NL (2015) Mechanisms of ammonia and ammonium transport by rhesus-associated glycoproteins. Am J Physiol Cell Physiol 309:C747–C758. https://doi.org/10.1152/ajpcell.00085.2015
Carattino MD, Montalbetti N (2020) Acid-sensing ion channels in sensory signaling. Am J Physiol Renal Physiol 318:F531–F543. https://doi.org/10.1152/ajprenal.00546.2019
Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hübner CA, Eladari D (2013) Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA 110:7928–7933. https://doi.org/10.1073/pnas.1221496110
Chen X, Kalbacher H, Gründer S (2006) Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol 127:267–276. https://doi.org/10.1085/jgp.200509409
Cheval L, Viollet B, Klein C, Rafael C, Figueres L, Devevre E, Zadigue G, Azroyan A, Crambert G, Vogt B, Doucet A (2021) Acidosis-induced activation of distal nephron principal cells triggers Gdf15 secretion and adaptive proliferation of intercalated cells. Acta Physiol (Oxf) 232:e13661. https://doi.org/10.1111/apha.13661
Christensen EI, Wagner CA, Kaissling B (2012) Uriniferous tubule: structural and functional organization. Compr Physiol 2:805–861. https://doi.org/10.1002/cphy.c100073
Cippà PE, Sun B, Liu J, Chen L, Naesens M, McMahon AP (2018) Transcriptional trajectories of human kidney injury progression. JCI Insight 3. https://doi.org/10.1172/jci.insight.123151
Cohen EP, Bastani B, Cohen MR, Kolner S, Hemken P, Gluck SL (1992) Absence of H(+)-ATPase in cortical collecting tubules of a patient with Sjogren’s syndrome and distal renal tubular acidosis. J Am Soc Nephrol 3:264–271. https://doi.org/10.1681/ASN.V32264
Corbet C, Feron O (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17:577–593. https://doi.org/10.1038/nrc.2017.77
Curthoys NP, Moe OW (2014) Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9:1627–1638. https://doi.org/10.2215/CJN.10391012
de Bruijn PIA, Larsen CK, Frische S, Himmerkus N, Praetorius HA, Bleich M, Leipziger J (2015) Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb. Am J Physiol Renal Physiol 309:F146–F153. https://doi.org/10.1152/ajprenal.00154.2015
Deyev IE, Sohet F, Vassilenko KP, Serova OV, Popova NV, Zozulya SA, Burova EB, Houillier P, Rzhevsky DI, Berchatova AA, Murashev AN, Chugunov AO, Efremov RG, Nikol’sky NN, Bertelli E, Eladari D, Petrenko AG (2011) Insulin receptor-related receptor as an extracellular alkali sensor. Cell Metab 13:679–689. https://doi.org/10.1016/j.cmet.2011.03.022
Dong B, Zhang X, Fan Y, Cao S, Zhang X (2017) GPR4 knockout improves renal ischemia–reperfusion injury and inhibits apoptosis via suppressing the expression of CHOP. Biochem J 474:4065–4074. https://doi.org/10.1042/BCJ20170676
DuBose TD (2000) Molecular and pathophysiologic mechanisms of hyperkalemic metabolic acidosis. Trans Am Clin Climatol Assoc 111:122–133; discussion 133–134
Eddy S, Mariani LH, Kretzler M (2020) Integrated multi-omics approaches to improve classification of chronic kidney disease. Nat Rev Nephrol 16:657–668. https://doi.org/10.1038/s41581-020-0286-5
Erra Díaz F, Ochoa V, Merlotti A, Dantas E, Mazzitelli I, Gonzalez Polo V, Sabatté J, Amigorena S, Segura E, Geffner J (2020) Extracellular acidosis and mTOR inhibition drive the differentiation of human monocyte-derived dendritic cells. Cell Rep 31:107613. https://doi.org/10.1016/j.celrep.2020.107613
Faivre A, Verissimo T, Auwerx H, Legouis D, de Seigneux S (2021) Tubular cell glucose metabolism shift during acute and chronic injuries. Front Med 8
Fitz R, Alsberg CL, Henderson LJ (1907) Concerning the excretion of phosphoric acid during experimental acidosis in rabbits. Am J Physiol 18:113–122. https://doi.org/10.1152/ajplegacy.1907.18.2.113
Fonseca F, Cerqueira R, Fuentes J (2019) Impact of ocean acidification on the intestinal microbiota of the marine sea bream (Sparus aurata L.). Front Physiol 10
Ghazi S, Bourgeois S, Gomariz A, Bugarski M, Haenni D, Martins JR, Nombela-Arrieta C, Unwin RJ, Wagner CA, Hall AM, Craigie E (2020) Multiparametric imaging reveals that mitochondria-rich intercalated cells in the kidney collecting duct have a very high glycolytic capacity. FASEB J 34:8510–8525. https://doi.org/10.1096/fj.202000273R
Gianella FG, Prado VE, Poindexter JR, Adams-Huet B, Li X, Miller RT, Sakhaee K, Maalouf NM, Moe OW (2021) Spot urinary citrate-to-creatinine ratio is a marker for acid-base status in chronic kidney disease. Kidney Int 99:208–217. https://doi.org/10.1016/j.kint.2020.07.006
Goraya N, Wesson DE (2017) Management of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis 24:298–304. https://doi.org/10.1053/j.ackd.2017.06.006
Goraya N, Simoni J, Sager LN, Madias NE, Wesson DE (2019) Urine citrate excretion as a marker of acid retention in patients with chronic kidney disease without overt metabolic acidosis. Kidney Int 95:1190–1196. https://doi.org/10.1016/j.kint.2018.11.033
Hage V, Villain C, Pelletier S, Laville M, Drai J, Fouque D (2019) Bicarbonate supplement restores urinary klotho excretion in chronic kidney disease: a pilot study. J Ren Nutr 29:285–288. https://doi.org/10.1053/j.jrn.2018.11.001
Hansen TH, Thomassen MT, Madsen ML, Kern T, Bak EG, Kashani A, Allin KH, Hansen T, Pedersen O (2018) The effect of drinking water pH on the human gut microbiota and glucose regulation: results of a randomized controlled cross-over intervention. Sci Rep 8:16626. https://doi.org/10.1038/s41598-018-34761-5
Harris AN, Grimm PR, Lee H-W, Delpire E, Fang L, Verlander JW, Welling PA, Weiner ID (2018) Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol 29:1411–1425. https://doi.org/10.1681/ASN.2017111163
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218. https://doi.org/10.1016/j.tibs.2014.02.002
Heering P, Ivens K, Aker S, Grabensee B (1998) Distal tubular acidosis induced by FK506. Clin Transplant 12:465–471
Henderson LJ (1908) The theory of neutrality regulation in the animal organism. Am J Physiol 21:427–448. https://doi.org/10.1152/ajplegacy.1908.21.4.427
Henderson LJ (1911) A critical study of the process of acid excretion1. J Biol Chem 9:403–424. https://doi.org/10.1016/S0021-9258(18)91456-0
Hernández J, Benedito JL, Abuelo A, Castillo C (2014) Ruminal acidosis in feedlot: from aetiology to prevention. Sci World J 2014:e702572. https://doi.org/10.1155/2014/702572
Hu MC, Kuro-o M, Moe OW (2013) Klotho and chronic kidney disease. Contrib Nephrol 180:47–63. https://doi.org/10.1159/000346778
Hultin S, Hood C, Campbell KL, Toussaint ND, Johnson DW, Badve SV (2021) A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep 6:695–705. https://doi.org/10.1016/j.ekir.2020.12.019
Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TAE, Gaston B (2000) Endogenous airway acidification. Am J Respir Crit Care Med 161:694–699. https://doi.org/10.1164/ajrccm.161.3.9911005
Imenez Silva PH, Wagner CA (2022) Physiological relevance of proton-activated GPCRs. Pflugers Arch 474:487–504. https://doi.org/10.1007/s00424-022-02671-1
Imenez Silva PH, Katamesh-Benabbas C, Chan K, Pastor Arroyo EM, Knöpfel T, Bettoni C, Ludwig M-G, Gasser JA, Brandao-Burch A, Arnett TR, Bonny O, Seuwen K, Wagner CA (2020) The proton-activated ovarian cancer G protein-coupled receptor 1 (OGR1) is responsible for renal calcium loss during acidosis. Kidney Int 97:920–933. https://doi.org/10.1016/j.kint.2019.12.006
Imenez Silva PH, Wiegand A, Daryadel A, Russo G, Ritter A, Gaspert A, Wüthrich RP, Wagner CA, Mohebbi N (2021) Acidosis and alkali therapy in patients with kidney transplant is associated with transcriptional changes and altered abundance of genes involved in cell metabolism and acid-base balance. Nephrol Dial Transplant 36:1806–1820. https://doi.org/10.1093/ndt/gfab210
Kaissling B (1982) Structural aspects of adaptive changes in renal electrolyte excretion. Am J Physiol Renal Physiol 243:F211–F226. https://doi.org/10.1152/ajprenal.1982.243.3.F211
Kaissling B (1985) Cellular heterogeneity of the distal nephron and its relation to function. Klin Wochenschr 63:868–876. https://doi.org/10.1007/BF01738139
Kajimoto S, Sakaguchi Y, Asahina Y, Kaimori J-Y, Isaka Y (2021) Modulation of the association of hypobicarbonatemia and incident kidney failure with replacement therapy by venous pH: a cohort study. Am J Kidney Dis 77:35–43. https://doi.org/10.1053/j.ajkd.2020.06.019
Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, Park ASD, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K (2015) Defective fatty acid oxidation in renal tubular epithelial cells plays a key role in kidney fibrosis development. Nat Med 21:37–46. https://doi.org/10.1038/nm.3762
Khacho M, Tarabay M, Patten D, Khacho P, MacLaurin JG, Guadagno J, Bergeron R, Cregan SP, Harper M-E, Park DS, Slack RS (2014) Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat Commun 5:3550. https://doi.org/10.1038/ncomms4550
Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, Goldring CEP, Powell H, Sanderson C, Williams S, Higgins L, Yamamoto M, Hayes J, Park BK (2010) Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteom 73:1612–1631. https://doi.org/10.1016/j.jprot.2010.03.018
Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SBG, Lazarenko RM, Ludwig M-G, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA (2015) Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348:1255–1260. https://doi.org/10.1126/science.aaa0922
LaMonte G, Tang X, Chen JL-Y, Wu J, Ding C-KC, Keenan MM, Sangokoya C, Kung H-N, Ilkayeva O, Boros LG, Newgard CB, Chi J-T (2013) Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab 1:23. https://doi.org/10.1186/2049-3002-1-23
Legouis D, Faivre A, Cippà PE, de Seigneux S (2020) Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol Dial Transplant gfaa302. https://doi.org/10.1093/ndt/gfaa302
Li S, Sato S, Yang X, Preisig P, Alpern R (2004) Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114:1782–1789
Li MM, White RR, Guan LL, Harthan L, Hanigan MD (2021) Metatranscriptomic analyses reveal ruminal pH regulates fiber degradation and fermentation by shifting the microbial community and gene expression of carbohydrate-active enzymes. Anim Microbiome 3:32. https://doi.org/10.1186/s42523-021-00092-6
Lightwood R (1935) British Paediatric Association. Arch Dis Child 10:205–210
Lister A, Bourgeois S, Silva PHI, Rubio-Aliaga I, Marbet P, Walsh J, Shelton LM, Keller B, Verrey F, Devuyst O, Giesbertz P, Daniel H, Goldring CE, Copple IM, Wagner CA, Odermatt A (2018) NRF2 regulates the glutamine transporter Slc38a3 (SNAT3) in kidney in response to metabolic acidosis. Sci Rep 8:5629. https://doi.org/10.1038/s41598-018-24000-2
Liu X, Liu J, Xiong K, Zhang C, Fang JK-H, Song J, Tai Z, Zhu Q, Hu M, Wang Y (2022) Effects of ocean acidification on molting, oxidative stress, and gut microbiota in juvenile horseshoe crab Tachypleus tridentatus. Front Physiol 12
López-Cayuqueo KI, Chavez-Canales M, Pillot A, Houillier P, Jayat M, Baraka-Vidot J, Trepiccione F, Baudrie V, Büsst C, Soukaseum C, Kumai Y, Jeunemaître X, Hadchouel J, Eladari D, Chambrey R (2018) A mouse model of pseudohypoaldosteronism type II reveals a novel mechanism of renal tubular acidosis. Kidney Int 94:514–523. https://doi.org/10.1016/j.kint.2018.05.001
Maclean AJ, Hayslett JP, Klein-Robbenhaar with the technical assistance of T, Myketey N (1980) Adaptive change in ammonia excretion in renal insufficiency. Kidney Int 17:595–606. doi: https://doi.org/10.1038/ki.1980.70
Mafra D, Borges NA, Lindholm B, Stenvinkel P (2019) Mitochondrial dysfunction and gut microbiota imbalance: an intriguing relationship in chronic kidney disease. Mitochondrion 47:206–209. https://doi.org/10.1016/j.mito.2018.11.006
Martini S, Nair V, Keller BJ, Eichinger F, Hawkins JJ, Randolph A, Böger CA, Gadegbeku CA, Fox CS, Cohen CD, Kretzler M (2014) Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol 25:2559–2572. https://doi.org/10.1681/ASN.2013080906
Marx D (2006) Proton transfer 200 years after von Grotthuss: insights from ab initio simulations. ChemPhysChem 7:1848–1870. https://doi.org/10.1002/cphc.200600128
Messa PG, Alfieri C, Vettoretti S (2016) Metabolic acidosis in renal transplantation: neglected but of potential clinical relevance. Nephrol Dial Transplant 31:730–736. https://doi.org/10.1093/ndt/gfv098
Mohebbi N, Mihailova M, Wagner CA (2009) The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol 297:F499–F509. https://doi.org/10.1152/ajprenal.90489.2008
Nagami GT, Hamm LL (2017) Regulation of acid-base balance in chronic kidney disease. Adv Chronic Kidney Dis 24:274–279. https://doi.org/10.1053/j.ackd.2017.07.004
Namba T, Takabatake Y, Kimura T, Takahashi A, Yamamoto T, Matsuda J, Kitamura H, Niimura F, Matsusaka T, Iwatani H, Matsui I, Kaimori J, Kioka H, Isaka Y, Rakugi H (2014) Autophagic clearance of mitochondria in the kidney copes with metabolic acidosis. J Am Soc Nephrol 25:2254–2266. https://doi.org/10.1681/ASN.2013090986
Nash TP, Benedict SR (1921) The ammonia content of the blood, and its bearing on the mechanism of acid neutralization in the animal organism. J Biol Chem 48:463–488. https://doi.org/10.1016/S0021-9258(18)86026-4
Nash TP, Williams EF (1932) Is blood protein amide nitrogen a source of urinary ammonia ? J Biol Chem 94:783–808. https://doi.org/10.1016/S0021-9258(18)76400-4
Nath KA, Hostetter MK, Hostetter TH (1985) Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76:667–675. https://doi.org/10.1172/JCI112020
Noble D (2012) A theory of biological relativity: no privileged level of causation. Interface Focus 2:55–64. https://doi.org/10.1098/rsfs.2011.0067
Olsen JSM, Svendsen S, Berg P, Dam VS, Sorensen MV, Matchkov VV, Leipziger J, Boedtkjer E (2021) NBCn1 increases NH4+ reabsorption across thick ascending limbs, the capacity for urinary NH4+ excretion, and early recovery from metabolic acidosis. J Am Soc Nephrol 32:852–865. https://doi.org/10.1681/ASN.2019060613
Packer RK, Desai SS, Hornbuckle K, Knepper MA (1991) Role of countercurrent multiplication in renal ammonium handling: regulation of medullary ammonium accumulation. J Am Soc Nephrol 2:77–83. https://doi.org/10.1681/ASN.V2177
Park S, Kang E, Park S, Kim YC, Han SS, Ha J, Kim DK, Kim S, Park S-K, Han DJ, Lim CS, Kim YS, Lee JP, Kim YH (2017) Metabolic acidosis and long-term clinical outcomes in kidney transplant recipients. J Am Soc Nephrol 28:1886–1897. https://doi.org/10.1681/ASN.2016070793
Pastor Arroyo EM, Yassini N, Sakiri E, Russo G, Bourgeois S, Mohebbi N, Amann K, Joller N, Wagner CA, Imenez Silva PH (2022) Alkali therapy protects renal function, suppresses inflammation, and improves cellular metabolism in kidney disease. Clin Sci (Lond) 136:557–577. https://doi.org/10.1042/CS20220095
Pattison LA, Callejo G, St John Smith E (2019) Evolution of acid nociception: ion channels and receptors for detecting acid. Phil Trans R Soc B 374:20190291. https://doi.org/10.1098/rstb.2019.0291
Pham TD, Elengickal AJ, Verlander JW, Al-Qusairi L, Chen C, Abood DC, King SA, Loffing J, Welling PA, Wall SM (2022) Pendrin-null mice develop severe hypokalemia following dietary Na+ and K+ restriction: role of ENaC. Am J Physiol Renal Physiol 322:F486–F497. https://doi.org/10.1152/ajprenal.00378.2021
Pitts RF (1975) Production of CO2 by the intact functioning kidney of the dog. Med Clin North Am 59:507–518. https://doi.org/10.1016/S0025-7125(16)32004-1
Pitts RF, Pilkington LA, MacLeod MB, Leal-Pinto E (1972) Metabolism of glutamine by the intact functioning kidney of the dog. Studies in metabolic acidosis and alkalosis. J Clin Invest 51:557–565
Preuss HG, Roxe DM, Bourke E (1987) Acidotic alterations in oxidative metabolism influencing rat renal slice amnoniagenesis. Life Sci 41:1695–1702. https://doi.org/10.1016/0024-3205(87)90596-0
Ray SC, Baban B, Tucker MA, Seaton AJ, Chang KC, Mannon EC, Sun J, Patel B, Wilson K, Musall JB, Ocasio H, Irsik D, Filosa JA, Sullivan JC, Marshall B, Harris RA, O’Connor PM (2018) Oral NaHCO3 activates a splenic anti-inflammatory pathway: evidence that cholinergic signals are transmitted via mesothelial cells. J Immunol 200:3568–3586. https://doi.org/10.4049/jimmunol.1701605
Riemann A, Wußling H, Loppnow H, Fu H, Reime S, Thews O (2016) Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochim Biophys Acta Mol Basis Dis 1862:72–81. https://doi.org/10.1016/j.bbadis.2015.10.017
Rossetti T, Jackvony S, Buck J, Levin LR (2021) Bicarbonate, carbon dioxide and pH sensing via mammalian bicarbonate-regulated soluble adenylyl cyclase. Interface Focus 11:20200034. https://doi.org/10.1098/rsfs.2020.0034
Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED (2001) Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98:4221–4226. https://doi.org/10.1073/pnas.071516798
Said MY, Rodriguez-Niño A, Post A, Schutten JC, Kieneker LM, Gomes-Neto AW, van Londen M, Osté MC, Borgonjen-van den Berg KJ, Nolte IM, van den Berg E, de Blaauw P, van der Krogt J, Heiner-Fokkema MR, Navis G, Yard BA, Bakker SJ (2021) Meat intake and risk of mortality and graft failure in kidney transplant recipients. Am J Clin Nutr 114:1505–1517. https://doi.org/10.1093/ajcn/nqab185
Savic Azoulay I, Liu F, Hu Q, Rozenfeld M, Ben Kasus Nissim T, Zhu MX, Sekler I, Xu T-L (2020) ASIC1a channels regulate mitochondrial ion signaling and energy homeostasis in neurons. J Neurochem 153:203–215. https://doi.org/10.1111/jnc.14971
Schwartz WB, Hall PW, Hays RM, Relman AS (1959) On the mechanisms of acidosis in chronic renal diesease*. J Clin Invest 38:39–52. https://doi.org/10.1172/JCI103794
Silverstein TP (2021) The proton in biochemistry: impacts on bioenergetics, biophysical chemistry, and bioorganic chemistry. Front Mol Biosci 8
Simpson DP (1971) Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 50:503–541. https://doi.org/10.1097/00005792-197111000-00002
Slyke DDV, Linder GC, Hiller A, Leiter L, McIntosh JF (1926) The excretion of ammonia and titratable acid in nephritis. J Clin Invest 2:255–288. https://doi.org/10.1172/JCI100045
Slyke DDV, Phillips RA, Hamilton PB, Archibald RM, Futcher PH, Hiller A (1943) Glutamine as source material of urinary ammonia. J Biol Chem 150:481–482. https://doi.org/10.1016/S0021-9258(18)72173-X
Sofi MH, Gudi R, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C (2014) pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes 63:632–644. https://doi.org/10.2337/db13-0981
Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE (2001) Pendrin: an apical Cl−/OH−/HCO3 −exchanger in the kidney cortex. Am J Physiol Renal Physiol 280:F356–F364. https://doi.org/10.1152/ajprenal.2001.280.2.F356
Song N, Lu Z, Zhang J, Shi Y, Ning Y, Chen J, Jin S, Shen B, Fang Y, Zou J, Teng J, Chu X-P, Shen L, Ding X (2019) Acid-sensing ion channel 1a is involved in ischaemia/reperfusion induced kidney injury by increasing renal epithelia cell apoptosis. J Cell Mol Med 23:3429–3440. https://doi.org/10.1111/jcmm.14238
Soriano JR (2002) Renal tubular acidosis: the clinical entity. J Am Soc Nephrol 13:2160–2170. https://doi.org/10.1097/01.ASN.0000023430.92674.E5
Steiner A, Goodman A, Treble D (1968) Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol 215:211–217. https://doi.org/10.1152/ajplegacy.1968.215.1.211
Stern L, Backman KA, Hayslett JP (1985) Effect of cortical-medullary gradient for ammonia on urinary excretion of ammonia. Kidney Int 27:652–661. https://doi.org/10.1038/ki.1985.60
Stettner P, Bourgeois S, Marsching C, Traykova-Brauch M, Porubsky S, Nordström V, Hopf C, Koesters R, Sandhoff R, Wiegandt H, Wagner CA, Gröne H-J, Jennemann R (2013) Sulfatides are required for renal adaptation to chronic metabolic acidosis. Proc Natl Acad Sci USA 110:9998–10003. https://doi.org/10.1073/pnas.1217775110
Stewart PA (1983) Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61:1444–1461
Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE (1997) Renal glucose production and utilization: new aspects in humans. Diabetologia 40:749–757. https://doi.org/10.1007/s001250050745
Su Y, Zhou A, Al-Lamki RS, Karet FE (2003) The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans*. J Biol Chem 278:20013–20018. https://doi.org/10.1074/jbc.M210077200
Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S (2010) Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol 21:1745–1755. https://doi.org/10.1681/ASN.2009050477
Sun X, Stephens L, DuBose TD, Petrovic S (2015) Adaptation by the collecting duct to an exogenous acid load is blunted by deletion of the proton-sensing receptor GPR4. Am J Physiol Renal Physiol 309:F120–F136. https://doi.org/10.1152/ajprenal.00507.2014
Tizianello A, Ferrari GD, Garibotto G, Gurreri G (1978) Effects of chronic renal insufficiency and metabolic acidosis on glutamine metabolism in man. Clin Sci Mol Med. https://doi.org/10.1042/CS0550391
van den Wildenberg MJ, Hoorn EJ, Mohebbi N, Wagner CA, Woittiez A-J, de Vries PAM, Laverman GD (2015) Distal renal tubular acidosis with multiorgan autoimmunity: a case report. Am J Kidney Dis 65:607–610. https://doi.org/10.1053/j.ajkd.2014.09.026
Van Slyke DD, Stillman E, Cullen GE (1919) Studies of acidosis: XIII. A method for titrating the bicarbonate content of the plasma. J Biol Chem 38:167–178. https://doi.org/10.1016/S0021-9258(18)87383-5
Wagner CA, Mohebbi N, Capasso G, Geibel JP (2011) The anion exchanger pendrin (SLC26A4) and renal acid-base homeostasis. Cell Physiol Biochem 28:497–504. https://doi.org/10.1159/000335111
Wagner CA, Imenez Silva PH, Bourgeois S (2019) Molecular pathophysiology of acid-base disorders. Semin Nephrol 39:340–352. https://doi.org/10.1016/j.semnephrol.2019.04.004
Wall SM, Lazo-Fernandez Y (2015) The role of pendrin in renal physiology. Annu Rev Physiol 77:363–378. https://doi.org/10.1146/annurev-physiol-021014-071854
Walsh S, Turner CM, Toye A, Wagner C, Jaeger P, Laing C, Unwin R (2007) Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant 22:807–812. https://doi.org/10.1093/ndt/gfl662
Warth R, Barrière H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J (2004) Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA 101:8215–8220. https://doi.org/10.1073/pnas.0400081101
Watanabe S, Tsuruoka S, Vijayakumar S, Fischer G, Zhang Y, Fujimura A, Al-Awqati Q, Schwartz GJ (2005) Cyclosporin A produces distal renal tubular acidosis by blocking peptidyl prolyl cis-trans isomerase activity of cyclophilin. Am J Physiol Renal Physiol 288:F40–F47. https://doi.org/10.1152/ajprenal.00218.2004
Weiner ID, Verlander JW (2011) Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300:F11–F23. https://doi.org/10.1152/ajprenal.00554.2010
Weinstein AM, Krahn TA (2010) A mathematical model of rat ascending Henle limb. II. Epithelial function. Am J Physiol Renal Physiol 298:F525–F542. https://doi.org/10.1152/ajprenal.00231.2009
Welbourne T, Weber M, Bank N (1972) The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest 51:1852–1860. https://doi.org/10.1172/JCI106987
Wesson DE (2021) The continuum of acid stress. Clin J Am Soc Nephrol. https://doi.org/10.2215/CJN.17541120
Wesson DE, Simoni J (2010) Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78:1128–1135. https://doi.org/10.1038/ki.2010.348
Wesson DE, Simoni J, Broglio K, Sheather S (2011) Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300:F830–F837. https://doi.org/10.1152/ajprenal.00587.2010
Wesson DE, Jo C-H, Simoni J (2015) Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant 30:762–770. https://doi.org/10.1093/ndt/gfu388
Wesson DE, Buysse JM, Bushinsky DA (2020) Mechanisms of metabolic acidosis–induced kidney injury in chronic kidney disease. J Am Soc Nephrol 31:469–482. https://doi.org/10.1681/ASN.2019070677
Wiegand A, Lim SF, von Moos S, Wüthrich RP, Held L, Mohebbi N (2021) Association of serum bicarbonate with graft survival and mortality in kidney transplant recipients. J Nephrol. https://doi.org/10.1007/s40620-021-01197-6
Wiegand A, Ritter A, Graf N, Arampatzis S, Sidler D, Hadaya K, Müller TF, Wagner CA, Wüthrich RP, Mohebbi N (2018) Preservation of kidney function in kidney transplant recipients by alkali therapy (Preserve-Transplant Study): rationale and study protocol. BMC Nephrol 19:177. https://doi.org/10.1186/s12882-018-0956-8
Wiegand A, Graf N, Bonani M, Frey D, Wüthrich RP, Mohebbi N (2019) Relationship of serum bicarbonate levels with 1-year graft function in kidney transplant recipients in Switzerland. Kidney Blood Press Res 44:1179–1188. https://doi.org/10.1159/000502527
Wolf KJ, Daft JG, Tanner SM, Hartmann R, Khafipour E, Lorenz RG (2014) Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J Histochem Cytochem 62:237–250. https://doi.org/10.1369/0022155413519650
Yeung SMH, Gomes-Neto AW, Osté MCJ, van den Berg E, Kootstra-Ros JE, Sanders JSF, Berger SP, Carrero JJ, Borst MHD, Navis GJ, Bakker SJL (2021) Net endogenous acid excretion and kidney allograft outcomes. Clin J Am Soc Nephrol 16:1398–1406. https://doi.org/10.2215/CJN.00780121
Yoon J, Liu Z, Lee E, Liu L, Ferre S, Pastor J, Zhang J, Moe OW, Chang AN, Miller RT (2021) Physiologic regulation of systemic klotho levels by renal CaSR signaling in response to CaSR ligands and pHo. J Am Soc Nephrol ASN.2021020276. https://doi.org/10.1681/ASN.2021020276
Yuli I, Oplatka A (1987) Cytosolic acidification as an early transductory signal of human neutrophil chemotaxis. Science 235:340–342. https://doi.org/10.1126/science.3798116
Zajac M, Dreano E, Edwards A, Planelles G, Sermet-Gaudelus I (2021) Airway surface liquid pH regulation in airway epithelium current understandings and gaps in knowledge. Int J Mol Sci 22:3384. https://doi.org/10.3390/ijms22073384
Zhao Y, Tarbell KV (2015) Comment on Sofi et al. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes 2014;63:632–644. Diabetes 64:e19. https://doi.org/10.2337/db15-0321
Acknowledgements
Work in the laboratory of the authors has been supported by the Swiss National Science Foundation (SNSF) to NM and through the SNSF-funded National Center of Competence in Research NCCR Kidney.CH to PHIS.
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PHIS declares no competing interests. NM has received speaker fees from Mundipharma and Boehringer Ingehlheim on subjects unrelated to this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Kidney Control of Homeostasis in Pflügers Archiv—European Journal of Physiology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imenez Silva, P.H., Mohebbi, N. Kidney metabolism and acid–base control: back to the basics. Pflugers Arch - Eur J Physiol 474, 919–934 (2022). https://doi.org/10.1007/s00424-022-02696-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02696-6