Abstract
The carbohydrate D-glucose is the main source of energy in living organisms. In contrast to animals, as well as most fungi, bacteria, and archaea, plants are capable to synthesize a surplus of sugars characterizing them as autothrophic organisms. Thus, plants are de facto the source of all food on earth, either directly or indirectly via feed to livestock. Glucose is stored as polymeric glucan, in animals as glycogen and in plants as starch. Despite serving a general source for metabolic energy and energy storage, glucose is the main building block for cellulose synthesis and represents the metabolic starting point of carboxylate- and amino acid synthesis. Finally yet importantly, glucose functions as signalling molecule conveying the plant metabolic status for adjustment of growth, development, and survival. Therefore, cell-to-cell and long-distance transport of photoassimilates/sugars throughout the plant body require the fine-tuned activity of sugar transporters facilitating the transport across membranes. The functional plant counterparts of the animal sodium/glucose transporters (SGLTs) are represented by the proton-coupled sugar transport proteins (STPs) of the plant monosaccharide transporter(-like) family (MST). In the framework of this special issue on “Glucose Transporters in Health and Disease,” this review gives an overview of the function and structure of plant STPs in comparison to the respective knowledge obtained with the animal Na+-coupled glucose transporters (SGLTs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Larger body size and an increase in complexity is a major trend in animal and plant evolution. Unicellular and multicellular organisms depend on diffusion to supply gases (oxygen, carbon dioxide) and nutrients as well as to remove toxic compounds. However, diffusion represents a slow process and works only across small distances (< 1 mm; [70]). With the appearance of organisms with a larger body size, the surface area cannot meet the needs of its volume in terms of nutrient and gas supply [34, 92]. To deliver essential substances to and from each cell in the body, the need arose to evolve internal transport systems to provide bulk flow transport.
In both, higher plants and animals, long-distance transport of carbohydrates is realized by a system of specialized tubes. In vertebrates, the vasculature forms a circulatory system of arteries, capillaries, and veins. In the closed circulatory system of vertebrates, blood circulates in the vascular system around and between all tissue layers in the body constantly driven by a pump—the heard. Thereby, the diffusion distance for gas, nutrient, and waste transport to individual cells is reduced. Pressure/flow rate, the composition of the blood, and last but not least, the glucose content are strictly controlled.
Plants do it differently
In plants, the situation is quite different: long-distance transport is not circulatory. More than 500 million years ago, the first plants conquered the land, and about 470 million years ago, the land plant lineage diverged into bryophytes (including liverworts and mosses) and vascular plants [71, 185]. In early land plants, long-distance transport occurs in cells arranged end-to-end in longitudinal files with a simplified cytoplasm and modified end walls. This design increased the intra- and intercellular conductivities for efficient bulk transport of nutrients, photoassimilates, and information [75, 76, 82, 102, 111, 116, 165]. The degree of specialization of conducting cells/tissues increased from non-vascular plants (bryophytes such as hornworts, mosses, and liverwort) to vascular cryptogams (early tracheophytes such as lycophytes and pterophytes) and finally to seed plants [75, 76, 82, 102, 116, 165]. Higher land plants (vascular plants) evolved vascular bundles with two distinct long-distance transport systems—the xylem and the phloem.
The xylem is a specialized plant tissue carrying water and dissolved minerals from the soil via the root to the shoot. This unidirectional transport pathway represents a continuous duct composed of tracheary elements, fibers, and parenchyma cells. Tracheary elements are dead cells with lignified secondary cell walls [97, 135]. These cells are elongated tubes with exaggerated ends that are interconnected through gaps in the cell wall (pits) with the ends of neibouring cells. Bulk flow of xylem sap is driven by transpiration at the leave surface utilizing the water potential difference between the soil and the atmosphere.
The phloem transports mainly photoassimilates, amino acids, and information in form of, e.g., phytohormones or small RNAs. The transport in the phloem is bidirectional originating from autotrophic sites where CO2 fixation takes place (source tissues) to heterotrophic tissues (sink tissues) such as developing leaves, meristems, roots, and reproductive organs that rely on an adequate sugar-supply by the phloem [55, 141, 165, 169, 170] (Fig. 1). The phloem network is assembled by highly active companion cells (CCs) and adjacent nucleus- and vacuole-free interconnected sieve elements (SEs) with increased intracellular conductivity for efficient bulk transport (Fig. 1). In apoplastically loading plants, the surplus of carbohydrates from source leaves are distributed in the plant throughout the vascular system (phloem) mainly in the form of the disaccharide sucrose (Fig. 1, [186]). Membrane-localized sucrose/H+ symporters (SUTs or SUCs) accumulate sucrose in the phloem cells in source tissues where sucrose can reach concentrations of up 1 M [15, 39, 41, 68, 77, 78, 118, 120, 137, 141, 142, 148, 149]. The hydrostatic pressure difference between source (sites of sugar accumulation) and sink (sites of sugar release) tissues drives the mass flow of water and nutrients in the phloem vessels [61, 96].
Long-distance transport of sucrose from source to sink tissues in apoplastically loading plants and the involvement of STPs in loading of sink cells with monosaccharides. Left: cartoon of a plant showing the phloem vasculature in dark green. The branched collecting phloem is illustrated only in the right fully developed leaf. Right: Illustration of the loading, the long-distance transport and the unloading of photoassimilates. In plants, photosynthetically synthesized sucrose is released from mesophyll cells to the apoplast (extracellular cell wall space) via SWEET type facilitators. At the source site of the phloem vasculature, H+-coupled sucrose transporters (SUTs) accumulate sucrose in the SE/CC (sieve element/companion cell complex—phloem tissue) complex for long-distance distribution throughout the plant body. H+-ATPase provide the proton motive force for sucrose loading energized by ATP hydrolysis. To provide heterotrophic sink cells with photoassimilates, sucrose is either imported symplastically via plasmodesmata or via a three-step apoplastic sugar import: (i) sucrose is released from the phloem cells into the apoplasm, (ii) cell wall-bound invertases hydrolyse sucrose to fructose and glucose, (iii) followed by the uptake of the breakdown products into sink cells via STP-type proton-coupled monosaccharide transporters
Why plants use sucrose instead of glucose for long-distance transport
As stated previously, long-distance transport of photoassimilates is mainly realized in the form of the disaccharide sucrose or less frequently, galactosides of sucrose (sugars of the raffinose family) [118, 186] and not in form of glucose as it is the case in animals. Sucrose is synthesized in photosynthetically active cells from fructose and glucose and is then transported via the phloem to heterotrophic parts of the plant. Sucrose transport is favorable for the following reasons: (i) 1 mol sucrose contains more energy than 1 mol of a monosaccharide such as glucose or fructose, thus using the disaccharide is more energy efficient for transport and storage. (ii) In contrast to fructose and glucose, sucrose represents a non-reducing sugar, meaning that it cannot be oxidized and thus no intermediate reactions with other molecules occur. High concentrations of reducing sugars and amino acid residues can undergo non-enzymatic glycosylation [85] leading to the impairment/damage of critical proteins [7, 159, 181]. Thus, plant cells sequester reducing sugars into the vacuole (Fig. 3) to avoid non-controllable glycosylation of cytosolic proteins [10]. During long-distance transport in living companion cells and sieve tubes of the phloem, sugar concentrations reach several hundred millimolar up to 1 M. The use of hexoses for transport at these high concentrations in the cytosol of phloem cells would lead to massive damages due to glycolysation. In vertebrates, long-distance transport is realized in the form of glucose extracellularly circulating in the bloodstream. The blood glucose concentration is tightly regulated to values in the low millimolar range (5.5 mmol/L global mean fasting plasma blood glucose level in humans; [25]). Thus, plants prefer the non-reducing sugar sucrose (or galactosides of sucrose) for long-distance transport [186]. In sink tissues, sucrose is converted back to glucose and fructose by an enzyme called (cell wall-bound) invertase (Fig. 1, [89, 121, 139, 167]). Subsequently, these hexoses are taken up by sink cells for consumption or storage. The uptake of hexoses across the plasma membrane is facilitated by a family of proton-coupled sugar transport proteins (STPs)—the counterpart of sodium-coupled glucose transporters of the SGLT-family in vertebrates. The function and the structure of these hexose-transporting proteins is the subject of this review.
Proton-coupled versus sodium-coupled secondary active transport
Transporters mediate the accumulation of substrates by coupling the substrate transport to a thermodynamically favorable symport or antiport of ions (usually Na+ or H+). Mitchell already in 1963 hypothesized the utilization of free energy stored in electrochemical ion gradients for substrate translocation [91]. ATP-driven primary active pumps—P-type H+-ATPases in the case of plants or Na+/K+-ATPases in animals—establish these electrochemical gradients across biological membranes [95, 104].
Plants utilize proton gradients for secondary active transport whereas animals prefer sodium gradients [98]. When eukaryotes diverged during evolution, plants—just like bacteria—continued using the abundant H+ ion to generate proton gradients via P-type H+-ATPases at the plasma membrane or vacuole-type ATPases as well as an H+-translocating pyrophosphatases at the vacuolar membrane [8, 33, 104, 110, 136, 138]. The activity of P-type proton pumps is important for membrane potential homeostasis and the strong hyperpolarization of plant membranes of more than – 200 mV [47]. Moreover, the accumulation of protons on the outside creates an inward-directed proton gradient with pH values of around 5.5 in the apoplast whereas the cytosolic pH is kept constant at around pH 7.5 [104, 157]. This proton motive force (proton gradients in conjunction with the hyperpolarized membrane potentials) are utilized by H+-coupled transporters for substrate transport/accumulation via H+/substrate symport or antiport mechanisms. Given a tight coupling between substrate and H+ transport, the free energy stored in the electrochemical proton gradient allows for a more than 1,000-fold accumulation of the substrate [20]. Animals evolved the Na+/K+ATPase for the maintenance of ionic gradients across the plasma membrane [49, 151, 152]. It creates ionic conditions/gradients for critical cellular processes such as secondary active transport of solutes and water, for pH regulation, and for creating and maintaining an electrical potential that is essential for the function of excitable cells in rapid signal transmission systems (e.g., muscles, nerves). Na+/K+ATPase does not exist in plants.
Phylogenie and physiology of monosaccharide transporters in plants
Higher plants express two gene families encoding for monosaccharide transporters: the cation/proton-coupled monosaccharide transporter(-like) superfamily (MSTs, [105, 117]) belonging to the major facilitator superfamily (MFS) and the recently identified SWEET transporter family (sugar will eventually be exported transporters) [23]—facilitators belonging to a group of transporters distinct from the MFS. An overview of the plant transport proteins and their classification into superfamilies/families is presented in Fig. 2a. The phylogenie and evolution of plant MSTs has comprehensively been reviewed by Johnson et al. previously [53, 54]. MSTs are integral membrane proteins that consist of 12 trans-membrane-spanning domains that assemble in a pseudosymmetrical manner to form a central pore/cavity shuttling soluble monosaccharides together with protons across hydrophobic membranes [18, 169]. In the model plant Arabidopsis thaliana, 53 members fulfil the criteria to represent a member of the MST-family [105, 117] (Fig. 2b). The Arabidopsis transporters of the MST-family divide into seven subfamilies that are conserved across the seed plants despite the variation in total number of family members in different species [53, 54]. Phylogenetic analysis using expressed sequence tag (EST) data showed that the MST gene family is ancient appearing at least 400 million years ago in land plants and showing differential subfamily expression and lineage-specific subfamily expansions [53]. The seven subfamilies of the plant MST-family (cf. Fig. 2b) consist of two large groups of subfamilies: EDR6 (early response to dehydration)-like and STP (sugar transport proteins) and five small subfamily groups: pGlcT/SBG1 (plastidic glucose transporter/suppressor of G protein beta1), INT (inositol or cyclic polyol transporters), PLT (polyol/monosaccharide transporters), AZT/TMT (tonoplastic monosaccharide transporters), and VGT (vacuolar glucose transporters). An overview of the subcellular localization of MST subfamilies is illustrated in Fig. 3. Although the members of the MST-family cluster into distinct subfamilies, several members exhibit overlapping expression patterns, subcellular localizations, and transport similar sugars. Moreover, many of these transporters transport more than one monosaccharide, though affinities for the substrates vary [17, 127, 140]. Only a few members of the MST(-like) superfamily have been shown to be specific for a single substrate such as AtSTP9 for glucose [131] and AtSTP14 for galactose [113].
Classification of plant transport proteins. a Plant transport processes comprise proteins for channel-mediated transport, carrier-mediated transport, and primary active transport. Secondary active sugar transport proteins are members of the major facilitator superfamily (MFS). The subfamily of monosaccharides transporters (MSTs) is further subdivided in seven groups of transporter families: EDR6 (early response to dehydration)-like, STP (sugar transport proteins), pGlcT/SBG1 (plastidic glucose transporter/Supressor of G protein beta1), INT (inositol or cyclic polyol transporters), PLT (polyol/monosaccharide transporters), AZT/TMT (tonoplastic monosaccharide transporters), and VGT (vacuolar glucose transporters). The family of disaccharide transporters (mainly sucrose transporters) constitute a distinct subfamily within the MFS—the GPH family. Monosaccharide and disaccharide facilitators of the SWEET (sugar will eventually be exported transporters) family are not members of the MFS but group into a distinct structural group of transporters. b Phylogenetic tree of the 53 members of MSTs in the model plant Arabidopsis thaliana. As mentioned in a, the MSTs are subdivided into 7 subfamilies
Cartoon illustrating a plant cell. Subcellular localization of MST subfamily groups is indicated. The subfamilies of the plant MST-family consist of EDR6 (early response to dehydration)-like, STP (sugar transport proteins), pGlcT/SBG1 (plastidic glucose transporter/Supressor of G protein beta1), INT (inositol or cyclic polyol transporters), PLT (polyol/monosaccharide transporters), AZT/TMT (tonoplastic monosaccharide transporters), and VGT (vacuolar glucose transporters)
Since the STP-subfamily is the best characterized plant subfamily of the MST family and since STPs represent the functional counterparts to human SGLTs, this review focuses on the STP-subfamily. The STP subfamily in the model plant Arabidopsis is encoded by 14 highly homologous STP genes [16] (Fig. 2b). AtSTPs have been functionally studied by heterologous expression in yeast or Xenopus oocytes and were shown to catalyse proton/sugar symport across the plasma membrane into the cell (see Table 1 for more information on functional properties of STPs; [16,17,18, 100, 123, 127, 131, 132, 134, 161] and references therein).
Although gene products of the plant MSTs cluster into phylogenetically distinct subfamilies (Fig. 2b), several transporters show overlapping expression patterns and transport similar sugars. Thus, genetic analyses of many single-gene knockouts of STPs fail to confer a significant phenotype indicating redundancy among similar transporters compensating the loss-of-function of a single transporter. Thus, linking the physiological role of individual STPs determined by heterologous expression to a specific function in plants appeared to be a major challenge. However, by the use of higher order knockouts generated by crossings and CRISPR/Cas9 or the overexpression of sugar transport proteins, major breakthroughs could be obtained [6, 122, 133].
As mentioned previously, photoassimilates are translocated from source to sink tissues over long distances mainly in form of sucrose [186]. Following the breakdown of sucrose to glucose and fructose via cell wall-bound invertases in the apoplastic space (Fig. 1), STPs are believed to transport the monosaccharides into sink cells, such as developing leaves, roots, pollen, flowers, and seeds [3, 17, 73, 87, 123, 128, 140].
Besides serving as a nutrient, glucose serves as a signalling molecule that is perceived by the enzyme Hexokinase1 (HXK1) in the cytosol of plant cells [50]. For example, pollen tube germination and growth require metabolic energy that has to be imported from the extracellular space across the plasma membrane in form of carbohydrates (Fig. 4a). Interestingly, however, pollen tube growth arrested upon glucose application [122]. This inhibitory effect was weakened in pollen tubes of a stp4-6-8-9-10-11 sextuple knockout Arabidopsis mutant as well as in the hxk1 knockout lines suggesting that STPs are responsible for glucose uptake into the growing pollen tube and that subsequently HXK1 detects the signalling molecule.
Cartoons illustrating the localization and function of STPs in various plant cell types. a In Arabidopsis pollen grain development and maturation in the anther of the flower involve the expression of AtSTP2, 4, 6, 9, and 11. Pollen grains germinate on the stigma. Pollen tubes enter the top of the pistil through the stigma and travel down the style to the ovules, which are contained in the ovary at the base of the pistil. AtSTP4, 10, and 11 are involved in pollen tube germination and growth. On the right, a pollen tube is shown in higher magnification. b Cartoon showing a rust infected plant leaf. Germination tubes of spores (S) at the leaf surface enter the leaves through stomata. Primary infection hyphae (IH) propagate through the leaf and penetrate the plant mesophyll cell (MC) wall but not the host plasma membrane to form a feeding structure—the haustorium. During infection, STP1, 4, and 13 expression and activity are induced as part of a defense response [9, 36, 178]. STPs are suggested to lower the apoplastic hexose concentration by moving the sugars into non-infected cells to limit the availability of extracellular saccharides for the pathogen. Another hypothesis implies that the pathogen hijackes STP13 to import sugars into the haustorium or import sugars into the cell that feeds the haustorium with sugars provided from adjacent non-infected cells (Yamada et al. 2016). c Guard cells embedded in the leaf epidermis regulate the stomatal opening for gas exchange (CO2-uptake, H2O release) between the plant and the atmosphere. In Arabidopsis guard cells, STP1 and 4 import mesophyll-derived glucose into guard cells for starch accumulation and light-induced stomatal opening. Thus, mesophyll-derived glucose uptake by guard cells connects photosynthesis with stomatal movements [35]
Besides supplying sink cells/tissues with sugars, the removal of sugar from the extracellular space has recently been shown to constitute a defense strategy against microbial infection by, e.g., powdery mildew and rust [9, 30, 74, 90, 93, 156, 179] (Fig. 4b). During infection, STP1, 4 and 13 expression, and activity are induced as part of the defense response [36, 179]. Thus, extracellular sugar depletion by STPs restricts the growth and development of microbial invaders (Fig. 4b). Another model suggests that biotrophic pathogens, such as fungi, may hijacke the plant hexose transporter STP13 to import sugars into the haustorium or import sugars into the cell that feeds the haustorium thereby increasing the availability of sugars in the infected cell [9, 179] (Fig. 4b).
Stomatal complexes—composed of two guard cells in the leaf epidermis—regulate the gas exchange (CO2-uptake, H2O release) between the plant and the atmosphere in response to various stimuli such as, e.g., light, drought, CO2 availability, and pathogens. In Arabidopsis, guard cells express the monosaccharide transporters STP1 and 4 (Fig. 4c). Flütsch et al could very recently show that STP1 and 4 are essential for light-induced stomatal opening and starch accumulation in guard cell. These transporters import mesophyll-derived glucose into guard cells connecting photosynthesis with stomatal movements [35].
Glucose transport in humans is accomplished by transporters encoded from two gene families. They are responsible for the absorption of glucose across the small intestine, the reabsorption of glucose from the glomerular filtrate, brain uptake across the blood–brain barrier, and the entire uptake and release of glucose from all kind of cells in the body. One family of glucose transporters facilitate the downhill, passive transport of glucose across cell membranes, the GLUT or SLC2 gene family [164]. Sodium-coupled uphill transport of glucose is mediated by a second gene family, the SGLT or SLC5 gene family [176].
Transport characteristics of plant monosaccharide transporters of the STP family
STP subfamily orthologs can already be found in the unicellular green algae Chlorella kessleri > 450 MYBP ago before Bryophytes evolved [22]. When Chlorella kessleri is grown under heterotrophic conditions, glucose serves as sole carbon source. Shifting the algae from carbon autotrophy to heterotrophy, hexose uptake increases more than 200-fold [44, 158]. The transport system for sugars has been associated with proteins encoded by the STP-like HUP (Hexose UPtake) genes (HUP1, 2, and 3). HUP transporters are members of the MFS-family of transporters just like the bacterial model transporter LacY—a H+-coupled lactose permease from Escherichia coli [56]. Although the helix packing seems identical to the one derived for the E. coli lac permease, the mechanism for transport and proton coupling seems to differ between the lac permease and the Chlorella symporter [22]. Detailed functional studies characterized HUP-transporters as H+-hexose symporters [62, 64,65,66], transporting sugars and protons with a stoichiometry of 1:1. Thereby, HUP1 accumulates substrates more than 1000-fold [63]. This accumulation has been explained by the tight coupling between H+ and sugar transport, distinct Km values for sugar uptake and release, and partly by differences of velocity constants contributing to uptake compared with those contributing to substrate release [63, 64]. These biochemical and biophysical studies were performed following functional HUP1 expression in different heterologous expression systems, such as yeast strains, Escherichia coli, Volvox carteri, and Xenopus oocytes [4, 126, 127].
Following the functional description of HUP genes from Chlorella, the hexose transporter STP1 from Arabidopsis represented the first cloned and characterized transporter of a higher plant [127]. Heterologous expression in the fission yeast Schizosaccharomyces pombe revealed that AtSTP1 transports glucose with high affinity (20 μM), but is also capable of transporting galactose, mannose, and xylose, however, at lower rates compared to glucose [17, 127]. Interestingly, only AtSTP6 and AtSTP13 transport fructose at significant rates [100, 134] while AtSTP9 is glucose specific [131]. For substrate specificities and affinities of further functionally characterized STP transporters, see Table 1, e.g., [16,17,18, 123, 124, 132, 161] and references therein). Only little is known about the mechanistic transport cycles of plant monosaccharide transporters. Concerning the kinetic mechanism of monosaccharide-H+ symport via STP-like transporters, AtSTP1 is the only member that has been studied in detail so far [11, 12]. Since kinetic parameters of membrane proteins often show pronounced voltage dependence, Borrer et al. (1994, [12]) expressed AtSTP1 in Xenopus oocytes. In contrast to yeast radiotracer flux analysis, heterologous expression in oocytes followed by two-electrode voltage clamp (TEVC) experiments provides the possibility to study kinetic parameter as a function of sugar and H+ concentrations in the membrane of a single cell under well-defined membrane potentials. AtSTP1 expressing oocytes were characterized by transport-associated glucose/3OMG (the non-metabolisable 3-O-methylglucose)-induced inward currents [11]. These transport-associated inward currents resemble the symport of protons (positively charged) with the uncharged sugar molecule. Thereby, K0.5-values of around 100 μM at pH 6 were calculated. This value in the low micromolar range is well in line with the affinity of AtSTP1 towards glucose determined in yeast (60 μM pH 6; [127]) and hexose uptake assays performed in higher plant protoplasts, cells, or tissue slices with Km-values in the range of 100–300 μM [115]. The high-affinity sugar uptake by STPs was later on further confirmed by sugar uptake studies in Arabidopsis seedlings comparing wild-type and an atstp1-KO mutant [139]. These data demonstrate that kinetic studies in heterologous expression systems can reflect the properties of sugar transporters in plants.
Furthermore, Borrer et al (1994, [12]) determined a Hill coefficient (n; cooperativity coefficient) for both protons and glucose of 1. n for glucose appeared independent from the external pH, suggesting that AtSTP1 transports H+ and glucose in a 1 to 1 stoichiometry. In order to further explore the molecular mechanism of AtSTP1 proton-coupled glucose transport and the underlying transport cycle, pre-steady-state currents (Ipre) of AtSTP1-expressing oocytes were measured (cf. [13, 21]). Most cation-coupled transporters characteristically display at least two main kinds of electrical currents: besides the organic substrate-associated ion translocation (transport-associated H+/Na+ currents, Itr), co-transporters exhibit pre-steady-state currents (Ipre, arising from charge movements in the electric field of the membrane) [45, 79, 107, 153]. While the pre-steady-state current is best observed in the absence of substrate, it disappears when the latter is present in saturating amounts [13, 81, 84, 101]. AtSTP1 pre-steady state currents displayed a similar behavior. In the absence of glucose and an external pH of 5.5, these transients could be described by an exponential equation and therein the decay rate constant (s) of Ipre and the quantity of displaced charges (Q) could be calculated. The observation that these transient currents vanished at saturating substrate concentrations or at neutral external pH values indicate that Ipre resemble the binding of protons to a site of AtSTP1 in the electrical field of the membrane or that conformational changes move charged residues that are located in the electrical field. Based on their kinetic studies, Boorer et al 1994 proposed a six-state transport cycle for AtSTP1 [12], where protons and sugars bind sequentially to the outward facing conformation of the transporter. The loaded transporter undergoes a conformational change presenting the binding sites for the sugar and protons to the cytosolic side of the membrane. Following the release of the substrates, the transporter reorientates in the membrane and thereby finalizes its transport cycle.
SGLT1, the functional animal counterpart of plant STPs, was also expressed and extensively studied in Xenopus oocytes using electron microscopic and optical methods as well as radioactive tracer flux analysis and electrophysiological techniques, summarized in [174]. In contrast to proton-coupled STP transporters, the rate and direction of sugar transport via SGLT1 was shown to depend on the magnitude and direction of the sodium electrochemical potential gradients with a fixed stoichiometry of 2 Na+ ions to 1 glucose molecule per transport cycle [31, 114, 162]. Interestingly, protons can drive glucose transport of SGLT1 as well—just like glucose transport via STPs—however, the affinity for sugar is about an order of magnitude lower than in the presence of sodium [173]. Vice versa, dependent on the individual transporter, the magnitude of transport currents but not the affinity towards the substrate of plant proton-coupled sucrose transporters seems to be influenced by high external sodium concentrations [183]. In the case of STPs, the influence of sodium on the hexose transport characteristics was not tested.
The K0.5 of SGLT1 for glucose and galactose is equal at 0.5 mM whereas SGLT2 seem to be glucose specific with a K0.5 of 2 mM. Just like a thermodynamically perfect machine, the transport direction of SGLT1 was fully reversible with a functional asymmetry between its cytosolic and extracellular face in respect to sugar affinity, sugar selectivity, and inhibitor susceptibility (phlorizin) [31, 106, 107, 114] cf. [20]. These studies revealed an essential ordered six-state kinetic model for Na/glucose cotransport by SGLT1. After the binding of two Na+ ions on the external surface, glucose binds to the outward-directed SGLT1 transporter. Due to a conformational change, sodium ions together with one glucose molecule are transported across the membrane, where glucose and the ions dissociate into the cytosol. The empty binding sites then re-orientate from the inner to the external surface to complete the transport cycle [107]. Thus, studies of AtSTP1 [12] and SGLT1 [107] in oocytes revealed a similar essential six-state model.
Plant sugar transporters of the MST family group into the same structural major facilitator superfamily (MFS; [125]) as the model transporters LacY, while the animal Na+/Glucose cotransporters SGLTs belong to the large sodium-solute symport family (SSF, [5]). Unfortunately, detailed biophysical and biochemical studies with STP-like transporters in respect to structure-function research, transport cycle, and the associated conformational change vanished in the 90th of the last century. In contrast, SGLT1 and LacY were intensively studied using the cysteine scanning mutagenesis approach in combination with functional studies to draw a mechanistic model of cation-coupled substrate transport across membranes (e.g., [2, 29, 37, 57, 79,80,81, 86, 146, 184]). Thereby, TM helices and/or individual amino acid residues were identified that contribute to sugar binding/selectivity that are accessible for functional fluorescent molecules such as tetramethyl-rhodamine-6-maleimide (TMRM6) or positions that lead to unfunctional SGLT/LacY mutants following the binding of –SH reagents to the introduced cysteine.
Site-directed alkylation and fluorescence-based methods, as well as single-molecule fluorescence resonance energy transfer (smFRET), support a six-state alternating access model, in which a conformational change is necessary for completion of the LacY transport catalysis [51, 86, 146]. In the case of the electrogenic SGLT1, electrophysiological measurements were combined with voltage-clamp fluorometry (VCF) in Xenopus laevis oocytes [79,80,81, 88]. Thereby, site-specific labeling of an introduced Cys residue with environmentally sensitive fluorophores enabled real-time observation of intramolecular movements under various conditions and positions (e.g., Q457 and G507) in the protein. These fluorescence studies of ligand-induced conformational changes in conjunction with pre-steady-state current analysis associated the charge movements (revealed from Ipre) with conformational changes of the sodium/glucose cotransporter. When SGLT1 was labelled at position 457, both the level and time course of the change in fluorescence (indicating movements of the specific protein domain) closely followed the charge movements that are a hallmark of the voltage-induced changes in state of the protein [79]. With SGLT1 labelled at position 507, the authors uncovered another slow conformational change probably due to a slow electroneutral step in the transport cycle associated with the release of sodium at the internal membrane surface [80]. Based on many studies using combinations of sophisticated electrophysiological and fluorescence-based techniques, an essential six-state reaction scheme with alternating access of the substrate binding sites for SGLT1 was proposed—similar to the situation in LacY [38, 59, 79,80,81, 107, 146].
Thus, VCF measurements represent a sophisticated technique to dissect the transport cycle of transporter proteins in partial reactions involved, e.g., in sugar binding and translocation. Neundlinger et al. could add further data in respect to glucose/inhibitor binding to SGLT1 in its natural environment using single-molecule force spectroscopy [99]. The authors resolved the forces and dynamics of glucose/inhibitor binding on the single-molecule level indicating that sugar translocation involves several steps with different temperature sensitivities.
Plant STP transporters were not studied in respect to conformational changes using VCF or similar techniques and thus data concerning transport-associated movements of the protein are not available. The only plant sugar transporter studied with VCF is ZmSUT1—a proton-coupled sucrose transporter originating from a distinct subfamily of the major facilitator superfamily (MFS)—the glycoside-pentoside-hexuronide (GPH) cation symporter family [27, 125] (Fig. 2a).
Structure of cation-coupled transporters from the MFS family
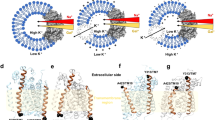
Today, the MFS superfamily includes millions of sequenced members distributed ubiquitously across all three kingdoms of life that transport a diverse variety of substrates in either uniport, symport, or antiport ([72, 105, 125, 182]; http://www.tcdb.org/). Until lately, information about the structure of plant sugar transporters was limited to sequence homologies to bacterial and animal cation-coupled transporters. These homologies with non-plant transporters supported the membership of plant hexose transporters in the major facilitator superfamily [105, 125]. In line with members of the MFS, hydropathy analyses with plant monosaccharide transporter sequences predicted 12 transmembrane helices with the N- and C-terminus located in the cytosol for the analyzed proteins (e.g., [119, 129]). Crystal structures of LacY (lactose/H+ permease from E. coli) and other MFS transporters elucidated the three-dimensional organization of this type of transporters ([2, 5] and references therein). It could be shown that these transporters consist of two pseudo-symmetrical halves formed by six TMD (transmembrane domains) bundles each, which are connected by an extended cytoplasmic loop. The sugar-binding site at the interface of the two halves is placed in the middle of the membrane. Biochemical and biophysical data resolved irreplaceable and highly conserved amino acid residues involved in substrate binding and proton transduction [58]. In high-resolution X-ray structures of LacY, a large internal cavity is open towards the cytoplasm but closed to the periplasm, representing the inward-facing conformation of the transporter [1, 42, 43]. Using double Gly-to-Trp mutants to introduce bulky side chains on the periplasmic side between the N- and C-terminal sixhelical halves of LacY [145] and later on with the help of nanobodies, the periplasmic-open conformation and transient conformers of LacY could be stabilized and crystalized [52, 69, 143, 144]. Thus, X-ray structures of LacY provide snapshots of local and global conformational changes that allow the sugar- and proton-binding sites gain alternating access to either side of the membrane (Fig. 5). These observations are underpinned by pre-steady-state kinetic analysis and multiple biochemical as well as spectroscopic approaches that—together with the structural data—document the alternating access mechanism underlying the symport of H+ and sugar in LacY (Fig. 5).
Alternating access model illustrated with the LacY structure. a Outward facing (accession number 4OAA) and b inward-facing (accession number 2Y5Y) structure of LacY. Structures are downloaded from PDB (www.pdb.org) and presented using UCSF ChimeraX (http://www.rbvi.ucsf.edu/chimerax, version 0.94). The helices were colored using rainbow from N (blue) to C terminus (red). Bound sugars are shown as spheres. The cytoplasmic surface is at the top
Based on these extensive studies and the high-resolution crystal structures, the LacY transporter often served as a template for 3D structural models of related transporters to predict tertiary structures and functional domains [40, 46, 108, 112, 166, 172, 187]. Sequence alignments and modelling attempts between LacY and plant sugar transporters showed only low sequence identities and only few of the essential amino acid site chains of LacY are present at identical positions in the respective TMDs of the plant counterparts. Thus, 3D modeling of plant sugar transporters may predict the overall tertiary structure only and will not explain the mechanism of high-affinity substrate transport in, e.g., STPs.
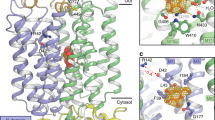
Very recently, Paulsen and co-workers determined the crystal structure of AtSTP10 in an outward facing occluded state with glucose bound in a central binding site at a resolution of 2.4 Å [109]. Just like AtSTP1, the pollen tube-expressed AtSTP10 represents a typical proton-driven symporter that besides glucose transports galactose and mannose with high affinity (low μM range, Table 1) [124]. The overall fold of AtSTP10 is reminiscent of the typical major facilitator structure with 12 TMDs divided into two pseudosymmetrical halves each consisting of six TM helices and a central sugar binding site located between the N- and C-terminal domains [109] (Fig. 6). Besides the common basic structural organization shared between the members of the MFS, the crystal structure of AtSTP10 revealed an unexpected feature: a “helix-helix-loop-helix” domain in the first extracellular loop between TMD1 and 2 that covers the entry pathway of sugars and was thus named “Lid” domain. This protrusion of the loop between TMD1-2 is covalently linked to the C-terminal domain by a disulfide bridge locking the N- and C-terminal domains together (Fig. 6). Due to the position of the Lid domain, there is no clear entry pathway for the substrate as seen in other major facilitators [26]. Interestingly, the Lid domain resembles a conserved feature found in sequences of all STPs and was thus far not found in any other member of the MFS.
A structural perspective of the Arabidopsis thaliana monosaccharide transporter AtSTP10 from side (a) and top (b) perspective. The structure represents an outward facing occluded state of the sugar transporter in complex with glucose (shown as spheres). Structure (accession number 6H7D) is downloaded from PDB (www.pdb.org) and presented using UCSF ChimeraX (http://www.rbvi.ucsf.edu/chimerax, version 0.94). The helices were colored using rainbow from N (blue) to C terminus (red). The surface of the STP-specific helix-helix-loop-helix motif (Lid) in the loop between TMD1-2 is illustrated in transparent blue. The cysteins involved in the disulfide bridge locking the N- and C-terminal domains together are shown as blue (Cys77) and orange (Cys449) spheres
How to explain the high affinity of STP transporters? Based on the crystal structure of AtSTP10, the authors found close interactions of glucose with three residues in the N-terminal domain (Phe39, Ile184, and Leu43) that create a hydrophobic interaction surface for the sugar. This tight and hydrophobic interaction surface cannot be found in low-affinity human GLUTs or bacterial sugar/H+ transporters, where the distance is longer and the interacting residues are polar [26, 48, 155]. High-affinity protein-ligand and protein-protein interactions seem to be based on tight hydrophobic interaction interfaces [60, 147] indicating that the high-affinity transport via STPs results from the tight binding of glucose to the identified hydrophobic interaction surface [109].
Below the Lid domain, only two charged residues (Asp42 and Arg142) were found in the TMD region that could act as proton donor/acceptor pair needed for proton translocation and the coupling between cation (H+) and sugar transport [14]. The position within the structure is similar to the respective position of other proton-coupled transporters of the MFS [48, 171] and mutants of AtSTP10 at positions Asp42 and Arg142 did not transport glucose anymore [109]. The authors suggest that protonation at Asp42 leads to a displacement of Arg 142 and thus a movement of the outer part of TMD1. This involves the movement of the glucose interaction sites Phe39 and Leu43, which subsequently leads to the tight coordination of the substrate. This scenario provides an explanation for the tight coupling of the proton-motive force and the transport of sugar. Due to the lack of structural information about the inward facing conformation of AtSTP10, the mechanism of sugar/H+ release and the accompanying conformational change remain to be elucidated.
Why does STPs possess a Lid domain? Based on their structural and functional findings, Paulsen et al. propose that the Lid domain via the disulfide bridge locks the N- and C-terminal six-helix bundles together (see Fig. 6) and that a conformational change must occur to allow the entry of the substrate to the binding site in the middle of the TMDs. The Lid isolates the proton donor/acceptor pair as well as the sugar-binding site from the extracellular environment and thereby creates a local milieu for efficient transport of glucose at physiological pH values of around pH 5 to 6. Respective mutants that do not possess a disulfide bridge between the Lid domain and the C-terminal domain displayed a pH optimum for transport of glucose that was markedly shifted to more acidic pH values.
In contrast to MFS members, SGLTs group into the large family of solute sodium symporters (SSS) [175, 176] that cotransport Na+ with sugars or in the case of SGLT3 act as glucose sensor [28, 130]. The physiological roles and the human diseases related to the disfunction of SGLT family members have been well characterized (detailed summaries of the pathophysiology of the SGLT family is the topic of other reviews within this issue). SGLTs share an alternating-access mechanism with tight coupling between Na+ and sugar transport [80, 83].
In 2008/2010, Faham et al. and Watanabe et al., respectively, [32, 168] could resolve the crystal structure of the Vibrio parahaemolyticus Na+/galactose symporter (vSGLT) with ~ 3.0 Å in the inward-facing occluded and the inward-facing open state. Experimental and in silico studies predicted 14 TMDs with extracellular amino and carboxy termini for SGLT1, which was finally confirmed by the crystal structure of vSGLT [162, 163]. The core structure consists of inverted repeats of 5 TMDs (TM2 to TM6 and TM7 to TM11) and galactose is bound in the center of the N-terminal and C-terminal 5 TMD bundles, occluded from the outside solutions by hydrophobic residues. Thus, despite of the similarity of the overall fold that is shared between vSGLT and LeuT (5+5) fold transporters (cf. [5]), vSGLT has its own specific structural modifications: vSGLT contains 14 TMDs and extracellular amino- and carboxyl-termini [32, 168]. Interestingly, the core structure of vSGLT is similar to the leucine transporter (LeuT) originating from a different gene family [67, 180]. This similarity, the vast amount of biophysical data of studies with SGLTs [99, 175], and the well-characterized structures of LeuT were sufficient to model the outward-facing confirmation of vSGLT and to predict sodium and sugar-binding sites as well as the structural movements accompanying the transport of Na+/sugars via SGLTs [32, 168].
Thus, having the structures from the plant AtSTP10 and its bacterial/animal counterpart in hands, it becomes clear that there are similarities but also pronounced differences among the different transporter families. These information could now help to perform detailed structure-function analysis to better understand the high-affinity transport mechanism of plant MSTs of the STP family.
Future opportunities
Pronounced progress in identifying, characterizing, and determining plant sugar transporters has been made within the last decades. However, in comparison to the deep biophysical, biochemical, and structural characterization of bacterial and animal model transporters, the plant functional orthologs have been studied in less detail so far. The high impact of basic research on bacterial and animal transport proteins, such as LacY, LeuT, SGLT, and GLUT, results at least in part from the incitement to understand the transport mechanisms in molecular/atomic detail to develop medications to cure diseases related to those transporters or transported substances. As sessil organisms, plants synthesize high numbers of various secondary metabolites; thus, plant transport proteins seem to be less sensitive to chemicals (agonists/antagonists/inhibitors) or alternatively, the pharmacology of plant transport proteins was not sufficiently tested so far. The incitement of the agricultural industry, funding agencies, and basic research at universities to understand plant transport proteins on the atomic level and subsequently to manipulate or protect crops from diseases seems to be less developed than the interest in animal/bacterial transporters.
With the first X-ray structure of a plant sugar transporter in hands (AtSTP10; [109]), the door is now open to combine biochemical and biophysical studies to understand the molecular mechanism of monosaccharide transport in plants. Therefore, further structural information about AtSTP10 in different conformations needs to be obtained to gain high-resolution snapshots of individual steps in its reaction cycle. These structural data have to be complemented with dynamic information about how sugar, protons, and the membrane potential induces local or global conformational changes and to understand the tight coupling between the proton motive force to the transport of sugar.
These studies have to include, e.g., site-directed mutagenesis, sophisticated electrophysiology, structural biology, molecular dynamics simulations, and fluorescence-based techniques to dissect the transport cycle of STP10 into individual steps. Thereby, the research on bacterial and animal transporters can guide the studies with plant transporters. The comparison of structural and functional features of different transporters will finally reveal fundamental principles of sugar transport with similarities and distinctions that (co-)evolved driven by specific challenges during the evolution of transport proteins in the three kingdoms of life.
Data availability
N/A
Change history
20 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00424-021-02603-5
References
Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S (2003) Structure and mechanism of the lactose permease of Escherichia coli. Science 301:610–615. https://doi.org/10.1126/science.1088196
Abramson J, Iwata S, Kaback HR (2004) Lactose permease as a paradigm for membrane transport proteins (Review). Mol Membr Biol 21:227–236. https://doi.org/10.1080/09687680410001716862
Afoufa-Bastien D, Medici A, Jeauffre J, Coutos-Thevenot P, Lemoine R, Atanassova R, Laloi M (2010) The Vitis vinifera sugar transporter gene family: phylogenetic overview and macroarray expression profiling. BMC Plant Biol 10:245. https://doi.org/10.1186/1471-2229-10-245
Aoshima H, Yamada M, Sauer N, Komor E, Schobert C (1993) Heterologous expression of the H+/Hexose cotransporter from chlorella in Xenopus oocytes and its characterization with respect to sugar specificity, pH and membrane-potential. J Plant Physiol 141:293–297. https://doi.org/10.1016/S0176-1617(11)81737-2
Bai X, Moraes TF, Reithmeier RAF (2017) Structural biology of solute carrier (SLC) membrane transport proteins. Mol Membr Biol 34:1–32. https://doi.org/10.1080/09687688.2018.1448123
Baier M, Hemmann G, Holman R, Corke F, Card R, Smith C, Rook F, Bevan MW (2004) Characterization of mutants in Arabidopsis showing increased sugar-specific gene expression, growth, and developmental responses. Plant Physiol 134:81–91. https://doi.org/10.1104/pp.103.031674
Bechtold U, Rabbani N, Mullineaux PM, Thornalley PJ (2009) Quantitative measurement of specific biomarkers for protein oxidation, nitration and glycation in Arabidopsis leaves. Plant J 59:661–671. https://doi.org/10.1111/j.1365-313X.2009.03898.x
Beyenbach KW, Wieczorek H (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209:577–589. https://doi.org/10.1242/jeb.02014
Bezrutczyk M, Yang JI, Eom JS, Prior M, Sosso D, Hartwig T, Szurek B, Oliva R, Vera-Cruz C, White FF, Yang B, Frommer WB (2018) Sugar flux and signaling in plant-microbe interactions. Plant J 93:675–685. https://doi.org/10.1111/tpj.13775
Boller T, Wiemken A (1986) Dynamics of vacuolar compartmentation. Annu Rev Plant Physiol 3:137–164
Boorer KJ, Forde BG, Leigh RA, Miller AJ (1992) Functional expression of a plant plasma membrane transporter in Xenopus oocytes. FEBS Lett 302:166–168. https://doi.org/10.1016/0014-5793(92)80431-f
Boorer KJ, Loo DD, Wright EM (1994) Steady-state and presteady-state kinetics of the H+/hexose cotransporter (STP1) from Arabidopsis thaliana expressed in Xenopus oocytes. J Biol Chem 269:20417–20424
Bossi E, Centinaio E, Castagna M, Giovannardi S, Vincenti S, Sacchi VF, Peres A (1999) Ion binding and permeation through the lepidopteran amino acid transporter KAAT1 expressed in Xenopus oocytes. J Physiol Lond 515:729–742. https://doi.org/10.1111/j.1469-7793.1999.729ab.x
Buch-Pedersen MJ, Pedersen BP, Veierskov B, Nissen P, Palmgren MG (2009) Protons and how they are transported by proton pumps. Pflugers Arch - Eur J Physiol 457:573–579. https://doi.org/10.1007/s00424-008-0503-8
Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118:59–68. https://doi.org/10.1104/pp.118.1.59
Buttner M (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581:2318–2324. https://doi.org/10.1016/j.febslet.2007.03.016
Buttner M (2010) The Arabidopsis sugar transporter (AtSTP) family: an update. Plant Biol (Stuttg) 12(Suppl 1):35–41. https://doi.org/10.1111/j.1438-8677.2010.00383.x
Buttner M, Sauer N (2000) Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta 1465:263–274. https://doi.org/10.1016/s0005-2736(00)00143-7
Büttner M, Truernit E, Baier K, Scholz-Starke J, Sontheim M, Lauterbach C, Huss VAR, Sauer N (2000) AtSTP3, a green leaf-specific, low affinity monosaccharide-H+ symporter of Arabidopsis thaliana. Plant Cell Environ 23:175–184. https://doi.org/10.1046/j.1365-3040.2000.00538.x
Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R (2005) Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem 280:21437–21443. https://doi.org/10.1074/jbc.M501785200
Carpaneto A, Koepsell H, Bamberg E, Hedrich R, Geiger D (2010) Sucrose- and H+-dependent charge movements associated with the gating of sucrose transporter ZmSUT1. PLoS One 5:ARTN e12605. https://doi.org/10.1371/journal.pone.0012605
Caspari T, Will A, Opekarova M, Sauer N, Tanner W (1994) Hexose/H+ symporters in lower and higher-plants. J Exp Biol 196:483–491
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532. https://doi.org/10.1038/nature09606
Cordoba E, Aceves-Zamudio DL, Hernandez-Bernal AF, Ramos-Vega M, Leon P (2015) Sugar regulation of sugar transporter protein 1 (STP1) expression in Arabidopsis thaliana. J Exp Bot 66:147–159. https://doi.org/10.1093/jxb/eru394
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang Y-H, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378:31–40. https://doi.org/10.1016/S0140-6736(11)60679-X
Deng D, Sun PC, Yan CY, Ke M, Jiang X, Xiong L, Ren WL, Hirata K, Yamamoto M, Fan SL, Yan N (2015) Molecular basis of ligand recognition and transport by glucose transporters. Nature 526:391. https://doi.org/10.1038/nature14655
Derrer C, Wittek A, Bamberg E, Carpaneto A, Dreyer I, Geiger D (2013) Conformational changes represent the rate-limiting step in the transport cycle of maize sucrose transporter1. Plant Cell 25:3010–3021. https://doi.org/10.1105/tpc.113.113621
Diez-Sampedro A, Wright EM, Hirayama BA (2001) Residue 457 controls sugar binding and transport in the Na(+)/glucose cotransporter. J Biol Chem 276:49188–49194. https://doi.org/10.1074/jbc.M108286200
Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H (2003) A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A 100:11753–11758. https://doi.org/10.1073/pnas.1733027100
Doidy J, Grace E, Kuhn C, Simon-Plas F, Casieri L, Wipf D (2012) Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci 17:413–422. https://doi.org/10.1016/j.tplants.2012.03.009
Eskandari S, Wright EM, Loo DDF (2005) Kinetics of the reverse mode of the Na+/glucose cotransporter. J Membr Biol 204:23–32. https://doi.org/10.1007/s00232-005-0743-x
Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J (2008) The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na(+)/sugar symport. Science 321:810–814. https://doi.org/10.1126/science.1160406
Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H(+)-ATPase regulation in the center of plant physiology. Mol Plant 9:323–337. https://doi.org/10.1016/j.molp.2015.11.002
Fisher SA, Burggren WW (2007) Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid Redox Signal 9:1339–1352. https://doi.org/10.1089/ars.2007.1704
Flutsch S, Nigro A, Conci F, Fajkus J, Thalmann M, Trtilek M, Panzarova K, Santelia D (2020) Glucose uptake to guard cells via STP transporters provides carbon sources for stomatal opening and plant growth. EMBO Rep e49719. https://doi.org/10.15252/embr.201949719
Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132:821–829. https://doi.org/10.1104/pp.103.021428
Frillingos S, Sahin-Toth M, Wu J, Kaback HR (1998) Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J 12:1281–1299. https://doi.org/10.1096/fasebj.12.13.1281
Gagnon DG, Frindel C, Lapointe JY (2007) Voltage-clamp fluorometry in the local environment of the C255-C511 disulfide bridge of the Na+/glucose cotransporter. Biophys J 92:2403–2411. https://doi.org/10.1529/biophysj.106.097964
Gahrtz M, Stolz J, Sauer N (1994) A phloem-specific sucrose-H+ symporter from Plantago-Major L supports the model of apoplastic phloem loading. Plant J 6:697–706. https://doi.org/10.1046/j.1365-313X.1994.6050697.x
Gottschalk KE, Soskine M, Schuldiner S, Kessler H (2004) A structural model of EmrE, a multi-drug transporter from Escherichia coli. Biophys J 86:3335–3348. https://doi.org/10.1529/biophysj.103.034546
Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci U S A 97:13979–13984. https://doi.org/10.1073/pnas.250473797
Guan L, Kaback HR (2007) Site-directed alkylation of cysteine to test solvent accessibility of membrane proteins. Nat Protoc 2:2012–2017. https://doi.org/10.1038/nprot.2007.275
Guan L, Mirza O, Verner G, Iwata S, Kaback HR (2007) Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A 104:15294–15298. https://doi.org/10.1073/pnas.0707688104
Haass D, Tanner W (1974) Regulation of hexose transport in Chlorella vulgaris: characteristics of induction and turnover. Plant Physiol 53:14–20. https://doi.org/10.1104/pp.53.1.14
Hilgemann DW, Lu CC (1999) GAT1 (GABA : Na+: Cl-) cotransport function—database reconstruction with an alternating access model. J Gen Physiol 114:459–475. https://doi.org/10.1085/jgp.114.3.459
Hirai T, Heymann JA, Shi D, Sarker R, Maloney PC, Subramaniam S (2002) Three-dimensional structure of a bacterial oxalate transporter. Nat Struct Biol 9:597–600. https://doi.org/10.1038/nsb821
Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280:918–921. https://doi.org/10.1126/science.280.5365.918
Iancu CV, Zamoon J, Woo SB, Aleshin A, Choe JY (2013) Crystal structure of a glucose/H+ symporter and its mechanism of action. Proc Natl Acad Sci U S A 110:17862–17867. https://doi.org/10.1073/pnas.1311485110
Jaitovich AA, Bertorello AM (2006) Na+, K+ -ATPase: an indispensable ion pumping-signaling mechanism across mammalian cell membranes. Semin Nephrol 26:386–392. https://doi.org/10.1016/j.semnephrol.2006.07.002
Jang JC, Leon P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9:5–19. https://doi.org/10.1105/tpc.9.1.5
Jiang X, Nie Y, Kaback HR (2011) Site-directed alkylation studies with LacY provide evidence for the alternating access model of transport. Biochemistry-Us 50:1634–1640. https://doi.org/10.1021/bi101988s
Jiang X, Smirnova I, Kasho V, Wu J, Hirata K, Ke M, Pardon E, Steyaert J, Yan N, Kaback HR (2016) Crystal structure of a LacY-nanobody complex in a periplasmic-open conformation. Proc Natl Acad Sci U S A 113:12420–12425. https://doi.org/10.1073/pnas.1615414113
Johnson DA, Thomas MA (2007) The monosaccharide transporter gene family in Arabidopsis and rice: a history of duplications, adaptive evolution, and functional divergence. Mol Biol Evol 24:2412–2423. https://doi.org/10.1093/molbev/msm184
Johnson DA, Hill JP, Thomas MA (2006) The monosaccharide transporter gene family in land plants is ancient and shows differential subfamily expression and expansion across lineages. BMC Evol Biol 6:64. https://doi.org/10.1186/1471-2148-6-64
Julius BT, Leach KA, Tran TM, Mertz RA, Braun DM (2017) Sugar transporters in plants: new insights and discoveries. Plant Cell Physiol 58:1442–1460. https://doi.org/10.1093/pcp/pcx090
Kaback HR (1997) A molecular mechanism for energy coupling in a membrane transport protein, the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A 94:5539–5543. https://doi.org/10.1073/pnas.94.11.5539
Kaback HR, Sahin-Toth M, Weinglass AB (2001) The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol 2:610–620. https://doi.org/10.1038/35085077
Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N (2007) Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A 104:491–494. https://doi.org/10.1073/pnas.0609968104
Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y (2011) The alternating access transport mechanism in LacY. J Membr Biol 239:85–93. https://doi.org/10.1007/s00232-010-9327-5
Kastritis PL, Bonvin AMJJ (2013) On the binding affinity of macromolecular interactions: daring to ask why proteins interact. J R Soc Interface 10:ARTN 20120835. https://doi.org/10.1098/rsif.2012.0835
Knoblauch M, Knoblauch J, Mullendore DL, Savage JA, Babst BA, Beecher SD, Dodgen AC, Jensen KH, Holbrook NM (2016) Testing the Munch hypothesis of long distance phloem transport in plants. Elife 5. https://doi.org/10.7554/eLife.15341
Komor E (1973) Proton-coupled hexose transport in Chlorella vulgaris. FEBS Lett 38:16–18. https://doi.org/10.1016/0014-5793(73)80501-0
Komor E, Tanner W (1974) The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol 64:568–581. https://doi.org/10.1085/jgp.64.5.568
Komor E, Tanner W (1974) The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem 44:219–223. https://doi.org/10.1111/j.1432-1033.1974.tb03476.x
Komor E, Tanner W (1976) The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem 70:197–204. https://doi.org/10.1111/j.1432-1033.1976.tb10970.x
Komor E, Haass D, Komor B, Tanner W (1973) Active hexose-uptake system of Chlorella-Vulgaris—Km-Values for 6-deoxyglucose influx and efflux and their contribution to sugar accumulation. Eur J Biochem 39:193–200. https://doi.org/10.1111/j.1432-1033.1973.tb03117.x
Krishnamurthy H, Gouaux E (2012) X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481:469–474. https://doi.org/10.1038/nature10737
Kuhn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275:1298–1300. https://doi.org/10.1126/science.275.5304.1298
Kumar H, Finer-Moore JS, Jiang X, Smirnova I, Kasho V, Pardon E, Steyaert J, Kaback HR, Stroud RM (2018) Crystal Structure of a ligand-bound LacY-Nanobody Complex. Proc Natl Acad Sci U S A 115:8769–8774. https://doi.org/10.1073/pnas.1801774115
LaBarbera M (1990) Principles of design of fluid transport systems in zoology. Science 249:992–1000. https://doi.org/10.1126/science.2396104
Lang D, Weiche B, Timmerhaus G, Richardt S, Riano-Pachon DM, Correa LG, Reski R, Mueller-Roeber B, Rensing SA (2010) Genome-wide phylogenetic comparative analysis of plant transcriptional regulation: a timeline of loss, gain, expansion, and correlation with complexity. Genome Biol Evol 2:488–503. https://doi.org/10.1093/gbe/evq032
Law CJ, Maloney PC, Wang DN (2008) Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol 62:289–305. https://doi.org/10.1146/annurev.micro.61.080706.093329
Lemoine R, La Camera S, Atanassova R, Dedaldechamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thevenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4:272. https://doi.org/10.3389/fpls.2013.00272
Lemonnier P, Gaillard C, Veillet F, Verbeke J, Lemoine R, Coutos-Thevenot P, La Camera S (2014) Expression of Arabidopsis sugar transport protein STP13 differentially affects glucose transport activity and basal resistance to Botrytis cinerea. Plant Mol Biol 85:473–484. https://doi.org/10.1007/s11103-014-0198-5
Ligrone R, Ducket JG, Renzaglia KS (2000) Conducting tissues and phyletic relationships of bryophytes. Philos Trans R Soc Lond Ser B Biol Sci 355:795–813. https://doi.org/10.1098/rstb.2000.0616
Ligrone R, Duckett JG, Renzaglia KS (2012) Major transitions in the evolution of early land plants: a bryological perspective. Ann Bot 109:851–871. https://doi.org/10.1093/aob/mcs017
Livingston DP, Henson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol 116:403–408. https://doi.org/10.1104/pp.116.1.403
Lohaus G, Hussmann M, Pennewiss K, Schneider H, Zhu JJ, Sattelmacher B (2000) Solute balance of a maize (Zea mays L.) source leaf as affected by salt treatment with special emphasis on phloem retranslocation and ion leaching. J Exp Bot 51:1721–1732. https://doi.org/10.1093/jexbot/51.351.1721
Loo DDF, Hazama A, Supplisson S, Turk E, Wright EM (1993) Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci U S A 90:5767–5771. https://doi.org/10.1073/pnas.90.12.5767
Loo DD, Hirayama BA, Gallardo EM, Lam JT, Turk E, Wright EM (1998) Conformational changes couple Na+ and glucose transport. Proc Natl Acad Sci U S A 95:7789–7794. https://doi.org/10.1073/pnas.95.13.7789
Loo DD, Hirayama BA, Karakossian MH, Meinild AK, Wright EM (2006) Conformational dynamics of hSGLT1 during Na+/glucose cotransport. J Gen Physiol 128:701–720. https://doi.org/10.1085/jgp.200609643
Lucas WJ, Groover A, Lichtenberger R, Furuta K, Yadav SR, Helariutta Y, He XQ, Fukuda H, Kang J, Brady SM, Patrick JW, Sperry J, Yoshida A, Lopez-Millan AF, Grusak MA, Kachroo P (2013) The plant vascular system: evolution, development and functions. J Integr Plant Biol 55:294–388. https://doi.org/10.1111/jipb.12041
Mackenzie B, Loo DDF, Wright EM (1998) Relationships between Na(+)/glucose cotransporter (SGLT1) currents and fluxes. J Membr Biol 162:101–106. https://doi.org/10.1007/s002329900347
Mager S, KleinbergerDoron N, Keshet GI, Davidson N, Kanner BI, Lester HA (1996) Ion binding and permeation at the GABA transporter GAT1. J Neurosci 16:5405–5414
Maillard L (1912) Action des acides amines sur les sucres: formation des melanoidines par voie methodologique. CR Hebd Acad Sci 154:66–68
Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR (2007) Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A 104:12640–12645. https://doi.org/10.1073/pnas.0700969104
McCurdy DW, Dibley S, Cahyanegara R, Martin A, Patrick JW (2010) Functional characterization and RNAi-mediated suppression reveals roles for hexose transporters in sugar accumulation by tomato fruit. Mol Plant 3:1049–1063. https://doi.org/10.1093/mp/ssq050
Meinild AK, Hirayama BA, Wright EM, Loo DD (2002) Fluorescence studies of ligand-induced conformational changes of the Na(+)/glucose cotransporter. Biochemistry-Us 41:1250–1258. https://doi.org/10.1021/bi011661r
Miller ME, Chourey PS (1992) The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell 4:297–305. https://doi.org/10.1105/tpc.4.3.297
Milne RJ, Dibley KE, Schnippenkoetter W, Mascher M, Lui ACW, Wang L, Lo C, Ashton AR, Ryan PR, Lagudah ES (2019) The wheat Lr67 gene from the sugar transport protein 13 family confers multipathogen resistance in barley. Plant Physiol 179:1285–1297. https://doi.org/10.1104/pp.18.00945
Mitchell P (1963) Molecule, group and electron translocation through the natural membranes. Biochem Soc Symp 22:141–168
Monahan-Earley R, Dvorak AM, Aird WC (2013) Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost 11(Suppl 1):46–66. https://doi.org/10.1111/jth.12253
Moore JW, Herrera-Foessel S, Lan C, Schnippenkoetter W, Ayliffe M, Huerta-Espino J, Lillemo M, Viccars L, Milne R, Periyannan S, Kong X, Spielmeyer W, Talbot M, Bariana H, Patrick JW, Dodds P, Singh R, Lagudah E (2015) A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat Genet 47:1494–1498. https://doi.org/10.1038/ng.3439
Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125:1074–1085. https://doi.org/10.1104/pp.125.2.1074
Morsomme P, Boutry M (2000) The plant plasma membrane H(+)-ATPase: structure, function and regulation. Biochim Biophys Acta 1465:1–16. https://doi.org/10.1016/s0005-2736(00)00128-0
Münch E (1930) Die stoffbewegungen in der pflanze. G. Fischer
Myburg A, Sederoff R (2001) Xylem structure and function. In: eLS. https://doi.org/10.1038/npg.els.0001302
Nagata T, Iizumi S, Satoh K, Kikuchi S (2008) Comparative molecular biological analysis of membrane transport genes in organisms. Plant Mol Biol 66:565–585. https://doi.org/10.1007/s11103-007-9287-z
Neundlinger I, Puntheeranurak T, Wildling L, Rankl C, Wang LX, Gruber HJ, Kinne RK, Hinterdorfer P (2014) Forces and dynamics of glucose and inhibitor binding to sodium glucose co-transporter SGLT1 studied by single molecule force spectroscopy. J Biol Chem 289:21673–21683. https://doi.org/10.1074/jbc.M113.529875
Norholm MH, Nour-Eldin HH, Brodersen P, Mundy J, Halkier BA (2006) Expression of the Arabidopsis high-affinity hexose transporter STP13 correlates with programmed cell death. FEBS Lett 580:2381–2387. https://doi.org/10.1016/j.febslet.2006.03.064
Nussberger S, Steel A, Trotti D, Romero MF, Boron WF, Hediger MA (1997) Symmetry of H+ binding to the intra- and extracellular side of the H+-coupled oligopeptide cotransporter PepT1. J Biol Chem 272:7777–7785. https://doi.org/10.1074/jbc.272.12.7777
Ohtani M, Akiyoshi N, Takenaka Y, Sano R, Demura T (2017) Evolution of plant conducting cells: perspectives from key regulators of vascular cell differentiation. J Exp Bot 68:17–26. https://doi.org/10.1093/jxb/erw473
Otori K, Tanabe N, Tamoi M, Shigeoka S (2019) Sugar transporter protein 1 (STP1) contributes to regulation of the genes involved in shoot branching via carbon partitioning in Arabidopsis. Biosci Biotechnol Biochem 83:472–481. https://doi.org/10.1080/09168451.2018.1550355
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845. https://doi.org/10.1146/annurev.arplant.52.1.817
Pao SS, Paulsen IT, Saier MH Jr (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62:1–34
Parent L, Supplisson S, Loo DD, Wright EM (1992) Electrogenic properties of the cloned Na+/glucose cotransporter: I. Voltage-clamp studies. J Membr Biol 125:49–62. https://doi.org/10.1007/BF00235797
Parent L, Supplisson S, Loo DDF, Wright EM (1992) Electrogenic properties of the cloned Na+ glucose cotransporter .2. A transport model under nonrapid equilibrium conditions. J Membr Biol 125:63–79
Patching SG, Baldwin SA, Baldwin AD, Young JD, Gallagher MP, Henderson PJ, Herbert RB (2005) The nucleoside transport proteins, NupC and NupG, from Escherichia coli: specific structural motifs necessary for the binding of ligands. Org Biomol Chem 3:462–470. https://doi.org/10.1039/b414739a
Paulsen PA, Custodio TF, Pedersen BP (2019) Crystal structure of the plant symporter STP10 illuminates sugar uptake mechanism in monosaccharide transporter superfamily. Nat Commun 10:407. https://doi.org/10.1038/s41467-018-08176-9
Pedersen CN, Axelsen KB, Harper JF, Palmgren MG (2012) Evolution of plant p-type ATPases. Front Plant Sci 3:31. https://doi.org/10.3389/fpls.2012.00031
Pittermann J (2010) The evolution of water transport in plants: an integrated approach. Geobiology 8:112–139. https://doi.org/10.1111/j.1472-4669.2010.00232.x
Popp C, Gorboulev V, Muller TD, Gorbunov D, Shatskaya N, Koepsell H (2005) Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Mol Pharmacol 67:1600–1611. https://doi.org/10.1124/mol.104.008839
Poschet G, Hannich B, Buttner M (2010) Identification and characterization of AtSTP14, a novel galactose transporter from Arabidopsis. Plant Cell Physiol 51:1571–1580. https://doi.org/10.1093/pcp/pcq100
Quick M, Tomasevic J, Wright EM (2003) Functional asymmetry of the human Na+/glucose transporter (hSGLT1) in bacterial membrane vesicles. Biochemistry-Us 42:9147–9152. https://doi.org/10.1021/bi034842x
Rausch T (1991) The hexose transporters at the plasma-membrane and the tonoplast of higher-plants. Physiol Plant 82:134–142
Raven JA (2003) Long-distance transport in non-vascular plants. Plant Cell Environ 26:73–85. https://doi.org/10.1046/j.1365-3040.2003.00920.x
Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH Jr (2012) The major facilitator superfamily (MFS) revisited. FEBS J 279:2022–2035. https://doi.org/10.1111/j.1742-4658.2012.08588.x
Rennie EA, Turgeon R (2009) A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci U S A 106:14162–14167. https://doi.org/10.1073/pnas.0902279106
Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11:4705–4713
Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13:1–7
Roitsch T, Tanner W (1994) Expression of a sugar-transporter gene family in a photoautotrophic suspension culture of Chenopodium rubrum L. Planta 193:365–371. https://doi.org/10.1007/BF00201814
Rottmann T, Zierer W, Subert C, Sauer N, Stadler R (2016) STP10 encodes a high-affinity monosaccharide transporter and is induced under low-glucose conditions in pollen tubes of Arabidopsis. J Exp Bot 67:2387–2399. https://doi.org/10.1093/jxb/erw048
Rottmann T, Fritz C, Sauer N, Stadler R (2018) Glucose uptake via STP transporters inhibits in vitro pollen tube growth in a hexokinase1-dependent manner in Arabidopsis thaliana. Plant Cell 30:2057–2081. https://doi.org/10.1105/tpc.18.00356
Rottmann T, Klebl F, Schneider S, Kischka D, Ruscher D, Sauer N, Stadler R (2018) Sugar transporter STP7 specificity for l-Arabinose and d-Xylose contrasts with the typical hexose transporters STP8 and STP12. Plant Physiol 176:2330–2350. https://doi.org/10.1104/pp.17.01493
Saier MH Jr, Tran CV, Barabote RD (2006) TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res 34:D181–D186. https://doi.org/10.1093/nar/gkj001
Sauer N, Stadler R (1993) A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum: cloning and heterologous expression in baker's yeast. Plant J 4:601–610. https://doi.org/10.1046/j.1365-313x.1993.04040601.x
Sauer N, Stolz J (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker’s yeast and identification of the histidine-tagged protein. Plant J 6:67–77. https://doi.org/10.1046/j.1365-313x.1994.6010067.x
Sauer N, Caspari T, Klebl F, Tanner W (1990) Functional expression of the Chlorella hexose transporter in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A 87:7949–7952. https://doi.org/10.1073/pnas.87.20.7949
Sauer N, Friedlander K, Graml-Wicke U (1990) Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J 9:3045–3050
Schafer N, Friedrich M, Jorgensen ME, Kollert S, Koepsell H, Wischmeyer E, Lesch KP, Geiger D, Doring F (2018) Functional analysis of a triplet deletion in the gene encoding the sodium glucose transporter 3, a potential risk factor for ADHD. PLoS One 13:e0205109. https://doi.org/10.1371/journal.pone.0205109
Schneidereit A, Scholz-Starke J, Buttner M (2003) Functional characterization and expression analyses of the glucose-specific AtSTP9 monosaccharide transporter in pollen of Arabidopsis. Plant Physiol 133:182–190. https://doi.org/10.1104/pp.103.026674
Schneidereit A, Scholz-Starke J, Sauer N, Buttner M (2005) AtSTP11, a pollen tube-specific monosaccharide transporter in Arabidopsis. Planta 221:48–55. https://doi.org/10.1007/s00425-004-1420-5
Schofield RA, Bi YM, Kant S, Rothstein SJ (2009) Over-expression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant Cell Environ 32:271–285. https://doi.org/10.1111/j.1365-3040.2008.01919.x
Scholz-Starke J, Buttner M, Sauer N (2003) AtSTP6, a new pollen-specific H+-monosaccharide symporter from Arabidopsis. Plant Physiol 131:70–77. https://doi.org/10.1104/pp.012666
Schuetz M, Smith R, Ellis B (2013) Xylem tissue specification, patterning, and differentiation mechanisms. J Exp Bot 64:11–31. https://doi.org/10.1093/jxb/ers287
Schumacher K (2006) Endomembrane proton pumps: connecting membrane and vesicle transport. Curr Opin Plant Biol 9:595–600. https://doi.org/10.1016/j.pbi.2006.09.001
Scofield GN, Hirose T, Aoki N, Furbank RT (2007) Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J Exp Bot 58:3155–3169. https://doi.org/10.1093/jxb/erm153
Segami S, Asaoka M, Kinoshita S, Fukuda M, Nakanishi Y, Maeshima M (2018) Biochemical, structural and physiological characteristics of vacuolar H+-pyrophosphatase. Plant Cell Physiol 59:1300–1308. https://doi.org/10.1093/pcp/pcy054
Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54:525–531. https://doi.org/10.1093/jxb/erg055
Slewinski TL (2011) Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: a physiological perspective. Mol Plant 4:641–662. https://doi.org/10.1093/mp/ssr051
Slewinski TL, Braun DM (2010) Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci 178:341–349. https://doi.org/10.1016/j.plantsci.2010.01.010
Slewinski TL, Meeley R, Braun DM (2009) Sucrose transporter1 functions in phloem loading in maize leaves. J Exp Bot 60:881–892. https://doi.org/10.1093/jxb/ern335
Smirnova IN, Kasho V, Kaback HR (2008) Protonation and sugar binding to LacY. Proc Natl Acad Sci U S A 105:8896–8901. https://doi.org/10.1073/pnas.0803577105
Smirnova I, Kasho V, Sugihara J, Kaback HR (2013) Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation. Proc Natl Acad Sci U S A 110:8876–8881. https://doi.org/10.1073/pnas.1306849110
Smirnova I, Kasho V, Jiang X, Pardon E, Steyaert J, Kaback HR (2014) Outward-facing conformers of LacY stabilized by nanobodies. Proc Natl Acad Sci U S A 111:18548–18553. https://doi.org/10.1073/pnas.1422265112
Smirnova I, Kasho V, Jiang X, Pardon E, Steyaert J, Kaback HR (2015) Transient conformers of LacY are trapped by nanobodies. Proc Natl Acad Sci U S A 112:13839–13844. https://doi.org/10.1073/pnas.1519485112
Snyder PW, Mecinovic J, Moustakas DT, Thomas SW, Harder M, Mack ET, Lockett MR, Heroux A, Sherman W, Whitesides GM (2011) Mechanism of the hydrophobic effect in the biomolecular recognition of arylsulfonamides by carbonic anhydrase. Proc Natl Acad Sci U S A 108:17889–17894. https://doi.org/10.1073/pnas.1114107108
Srivastava AC, Ganesan S, Ismail IO, Ayre BG (2008) Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol 148:200–211. https://doi.org/10.1104/pp.108.124776
Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N (1995) Phloem loading by the Pmsuc2 sucrose carrier from Plantago-Major occurs into companion cells. Plant Cell 7:1545–1554
Stadler R, Buttner M, Ache P, Hedrich R, Ivashikina N, Melzer M, Shearson SM, Smith SM, Sauer N (2003) Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol 133:528–537. https://doi.org/10.1104/pp.103.024240
Stein WD (1995) The sodium pump in the evolution of animal cells. Philos Trans R Soc Lond Ser B Biol Sci 349:263–269. https://doi.org/10.1098/rstb.1995.0112
Stein WD (2002) Cell volume homeostasis: ionic and nonionic mechanisms. The sodium pump in the emergence of animal cells. Int Rev Cytol 215:231–258. https://doi.org/10.1016/s0074-7696(02)15011-x
Su A, Mager S, Mayo SL, Lester HA (1996) A multi-substrate single-file model for ion-coupled transporters. Biophys J 70:762–777. https://doi.org/10.1016/S0006-3495(96)79616-9
Subtil T, Boles E (2011) Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol Biofuels 4:38. https://doi.org/10.1186/1754-6834-4-38
Sun LF, Zeng X, Yan CY, Sun XY, Gong XQ, Rao Y, Yan NE (2012) Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490:361. https://doi.org/10.1038/nature11524
Sutton PN, Gilbert MJ, Williams LE, Hall JL (2007) Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Physiol Plant 129:787–795. https://doi.org/10.1111/j.1399-3054.2007.00863.x
Sze H (1985) H-translocating ATPase: advances using membrane vesicles. Annu Rev Plant Physiol 36:175–208
Tanner W (1969) Light-driven active uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Commun 36:278–283. https://doi.org/10.1016/0006-291x(69)90326-x
Thornalley PJ (2003) Glyoxalase I: structure, function and a critical role in the enzymatic defense against glycation. Biochem Soc Trans 31:1343–1348
Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8:2169–2182. https://doi.org/10.1105/tpc.8.12.2169
Truernit E, Stadler R, Baier K, Sauer N (1999) A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J 17:191–201. https://doi.org/10.1046/j.1365-313x.1999.00372.x
Turk E, Wright EM (1997) Membrane topology motifs in the SGLT cotransporter family. J Membr Biol 159:1–20. https://doi.org/10.1007/s002329900264
Turk E, Kim O, le Coutre J, Whitelegge JP, Eskandari S, Lam JT, Kreman M, Zampighi G, Faull KF, Wright EM (2000) Molecular characterization of Vibrio parahaemolyticus vSGLT—a model for sodium-coupled sugar cotransporters. J Biol Chem 275:25711–25716. https://doi.org/10.1074/jbc.M003127200
Uldry M, Thorens B (2004) The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch - Eur J Physiol 447:480–489. https://doi.org/10.1007/s00424-003-1085-0
Van Bel AJE (2003) The phloem, a miracle of ingenuity. Plant Cell Environ 26:125–149. https://doi.org/10.1046/j.1365-3040.2003.00963.x
Vardy E, Arkin IT, Gottschalk KE, Kaback HR, Schuldiner S (2004) Structural conservation in the major facilitator superfamily as revealed by comparative modeling. Protein Sci 13:1832–1840. https://doi.org/10.1110/ps.04657704
von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9:3033–3044
Watanabe A, Choe S, Chaptal V, Rosenberg JM, Wright EM, Grabe M, Abramson J (2010) The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature 468:988–U162. https://doi.org/10.1038/nature09580
Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants—a diversity of roles and complex regulation. Trends Plant Sci 5:283–290. https://doi.org/10.1016/s1360-1385(00)01681-2
Wind J, Smeekens S, Hanson J (2010) Sucrose: metabolite and signaling molecule. Phytochemistry 71:1610–1614. https://doi.org/10.1016/j.phytochem.2010.07.007
Wisedchaisri G, Park MS, Iadanza MG, Zheng H, Gonen T (2014) Proton-coupled sugar transport in the prototypical major facilitator superfamily protein XylE. Nat Commun 5:4521. https://doi.org/10.1038/ncomms5521
Wood JM, Culham DE, Hillar A, Vernikovska YI, Liu F, Boggs JM, Keates RAB (2005) A structural model for the osmosensor, transporter, and osmoregulator ProP of Escherichia coli. Biochemistry-Us 44:5634–5646. https://doi.org/10.1021/bi047383o
Wright EM, Turk E (2004) The sodium/glucose cotransport family SLC5. Pflugers Arch - Eur J Physiol 447:510–518. https://doi.org/10.1007/s00424-003-1063-6
Wright EMLD, Hirayama BA, Turk E (2006) Sugar absorption. In: Johnson L (ed) Physiology of the Gastrointestinal Tract, 4th edn. Elsevier/Academic Press, San Diego
Wright EM, Hirayama BA, Loo DF (2007) Active sugar transport in health and disease. J Intern Med 261:32–43. https://doi.org/10.1111/j.1365-2796.2006.01746.x
Wright EM, Loo DD, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91:733–794. https://doi.org/10.1152/physrev.00055.2009
Yamada K, Kanai M, Osakabe Y, Ohiraki H, Shinozaki K, Yamaguchi-Shinozaki K (2011) Monosaccharide absorption activity of Arabidopsis roots depends on expression profiles of transporter genes under high salinity conditions. J Biol Chem 286:43577–43586. https://doi.org/10.1074/jbc.M111.269712
Yamada K, Saijo Y, Nakagami H, Takano Y (2016) Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 354:1427–1430. https://doi.org/10.1126/science.aah5692
Yamada K, Osakabe Y, Yamaguchi-Shinozaki K (2017) A C-terminal motif contributes to the plasma membrane localization of Arabidopsis STP transporters. PLoS One 12:e0186326. https://doi.org/10.1371/journal.pone.0186326
Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E (2005) Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature 437:215–223. https://doi.org/10.1038/nature03978
Yamauchi Y, Ejiri Y, Tanaka K (2002) Glycation by ascorbic acid causes loss of activity of ribulose-1,5-bisphosphate carboxylase/oxygenase and its increased susceptibility to proteases. Plant Cell Physiol 43:1334–1341. https://doi.org/10.1093/pcp/pcf162
Zhang XC, Zhao Y, Heng J, Jiang D (2015) Energy coupling mechanisms of MFS transporters. Protein Sci 24:1560–1579. https://doi.org/10.1002/pro.2759
Zhou JJ, Miller AJ (2000) Comparison of the transport properties of three plant sucrose carriers expressed in Xenopus oocytes. Aust J Plant Physiol 27:725–732
Zhou Y, Guan L, Freites JA, Kaback HR (2008) Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci U S A 105:3774–3778. https://doi.org/10.1073/pnas.0800825105
Zimmer A, Lang D, Richardt S, Frank W, Reski R, Rensing SA (2007) Dating the early evolution of plants: detection and molecular clock analyses of orthologs. Mol Gen Genomics 278:393–402. https://doi.org/10.1007/s00438-007-0257-6
Zimmermann M, Ziegler H (1975) List of sugar and sugar alcohols in sieve-tube exudates. Encycl Plant Physiol:479–503
Zuniga FA, Shi GP, Haller JF, Rubashkin A, Flynn DR, Iserovich P, Fischbarg J (2001) A three-dimensional model of the human facilitative glucose transporter Glut1. J Biol Chem 276:44970–44975. https://doi.org/10.1074/jbc.M107350200
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the Deutsche Forschungsgemeinschaft (DFG) for grants within the project GE2195/1-1.
Author information
Authors and Affiliations
Contributions
N/A
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflicts of interest.
Ethics approval
N/A
Consent to participate
N/A
Consent for publication
N/A
Code availability
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
This article is published as part of the Special Issue on “Glucose Transporters in Health and Disease”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geiger, D. Plant glucose transporter structure and function. Pflugers Arch - Eur J Physiol 472, 1111–1128 (2020). https://doi.org/10.1007/s00424-020-02449-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02449-3