Abstract
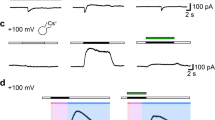
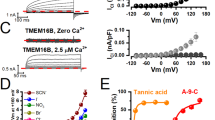
A novel type of anion channel activated by extracellular acidification, called acid-sensitive outwardly rectifying (ASOR) anion channel, was shown to be involved in acidotoxic necrotic death in human epithelial cells. However, its biophysical property and molecular identity have remained elusive. In human epithelial HeLa cells, here, whole-cell currents of ASOR anion channel were found to be augmented by warm temperature, with a threshold temperature of 32 °C. Temperature sensitivity of the conductance was found to be high (with Q 10 of 8.8) in the range of body temperature, suggesting a possible involvement of a non-diffusion-limited process such as a transporter-mediated conduction. In this regard, it is interesting that a Cl−/H+ antiporter ClC-3 has recently been proposed as a candidate for the ASOR channel. However, siRNA-mediated knockdown of hClC-3 failed to suppress ASOR currents in HeLa cells. Also, endogenous ASOR currents in HEK293T cells were not affected by overexpression of human or mouse ClC-3. Furthermore, functional expression of the ASOR channel was virtually absent in the cisplatin-resistant human cancer KCP-4 cell line despite the fact that molecular expression of ClC-3 was indistinguishable between KCP-4 cells and parental cisplatin-sensitive KB-3-1 cells which endogenously exhibit high activity of ASOR anion channels. These results indicate that the ASOR anion channel is highly sensitive to temperature and independent of ClC-3.






Similar content being viewed by others
References
Auzanneau C, Thoreau V, Kitzis A, Becq F (2003) A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat sertoli cells. J Biol Chem 278:19230–19236. doi:10.1074/jbc.M301096200
Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R (2006) A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 26:4835–4840. doi:10.1523/jneurosci.5080-05.2006
Chen MF, Chen TY (2001) Different fast-gate regulation by external Cl− and H+ of the muscle-type ClC chloride channels. J Gen Physiol 118:23–32. doi:10.1085/jgp.118.1.23
Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, Raouf R, Wood JN, Oh U (2012) The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci 15:1015–1021. doi:10.1038/nn.3111
Diewald L, Rupp J, Dreger M, Hucho F, Gillen C, Nawrath H (2002) Activation by acidic pH of CLC-7 expressed in oocytes from Xenopus laevis. Biochem Biophys Res Commun 291:421–424. doi:10.1006/bbrc.2002.6462
Duan D, Winter C, Cowley S, Hume JR, Horowitz B (1997) Molecular identification of a volume-regulated chloride channel. Nature 390:417–421. doi:10.1038/37151
Fujii R, Mutoh M, Niwa K, Yamada K, Aikou T, Nakagawa M, Kuwano M, Akiyama S (1994) Active efflux system for cisplatin in cisplatin-resistant human KB cells. Jpn J Cancer Res 85:426–433
Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:6408–6414
Gees M, Colsoul B, Nilius B (2010) The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2:a003962. doi:10.1101/cshperspect.a003962
Hille B (2001) Ion channels of excitable membranes, 3rd edn. Sinauer, Sunderland
Jentsch TJ (2008) CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43:3–36. doi:10.1080/10409230701829110
Jordt SE, Jentsch TJ (1997) Molecular dissection of gating in the ClC-2 chloride channel. EMBO J 16:1582–1592. doi:10.1093/emboj/16.7.1582
Kajiya H, Okamoto F, Ohgi K, Nakao A, Fukushima H, Okabe K (2009) Characteristics of ClC7 Cl− channels and their inhibition in mutant (G215R) associated with autosomal dominant osteopetrosis type II in native osteoclasts and hClcn7 gene-expressing cells. Pflugers Arch 458:1049–1059. doi:10.1007/s00424-009-0689-4
Lambert S, Oberwinkler J (2005) Characterization of a proton-activated, outwardly rectifying anion channel. J Physiol 567:191–213. doi:10.1113/jphysiol.2005.089888
Matsuda JJ, Filali MS, Collins MM, Volk KA, Lamb FS (2010) The ClC-3 Cl−/H+ antiporter becomes uncoupled at low extracellular pH. J Biol Chem 285:2569–2579. doi:10.1074/jbc.M109.018002
Matsuda JJ, Filali MS, Moreland JG, Miller FJ, Lamb FS (2010) Activation of swelling-activated chloride current by tumor necrosis factor-α requires ClC-3-dependent endosomal reactive oxygen production. J Biol Chem 285:22864–22873. doi:10.1074/jbc.M109.099838
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Nilius B, Voets T (2005) TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch 451:1–10. doi:10.1007/s00424-005-1462-y
Nobles M, Higgins CF, Sardini A (2004) Extracellular acidification elicits a chloride current that shares characteristics with I Cl(swell). Am J Physiol Cell Physiol 287:C1426–C1435. doi:10.1152/ajpcell.00549.2002
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol 273:C755–C789
Okada Y, Sato K, Toychiev AH, Suzuki M, Dutta AK, Inoue H, Sabirov RZ (2009) The puzzles of volume-activated anion channels. In: Alvarez-Leefmans FJ, Delpire E (eds) Physiology and pathology of chloride transporters and channels in the nervous system. From molecules to diseases. Elsevier, San Diego, pp 283–306
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049. doi:10.1126/science.1073140
Sato K, Numata T, Lee EL, Okada Y (2012) Exploration of the molecular identity of acid-sensitive outwardly rectifying Cl− channel (ASOR). J Physiol Sci 62(Suppl 1):S84
Stroffekova K, Kupert EY, Malinowska DH, Cuppoletti J (1998) Identification of the pH sensor and activation by chemical modification of the ClC-2G Cl− channel. Am J Physiol Cell Physiol 275:C1113–C1123
Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25:1804–1815. doi:10.1038/sj.emboj.7601083
Wang H-Y, Shimizu T, Numata T, Okada Y (2007) Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflugers Arch 454:223–233. doi:10.1007/s00424-006-0193-z
Wang L, Ma W, Zhu L, Ye D, Li Y, Liu S, Li H, Zuo W, Li B, Ye W, Chen L (2012) ClC-3 is a candidate of the channel proteins mediating acid-activated chloride currents in nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol 303:C14–C23. doi:10.1152/ajpcell.00145.2011
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–186
Yamamoto S, Ehara T (2006) Acidic extracellular pH-activated outwardly rectifying chloride current in mammalian cardiac myocytes. Am J Physiol Heart Circ Physiol 290:H1905–H1914. doi:10.1152/ajpheart.00965.2005
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from JSPS and MEXT. The authors thank K. Shigemoto and N. Yasui for technical assistance, and T. Okayasu for secretarial assistance. We also thank the Functional Genomics Facility at the National Institute for Basic Biology for allowing the use of equipments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kaori Sato-Numata and Tomohiro Numata are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 252 kb)
Rights and permissions
About this article
Cite this article
Sato-Numata, K., Numata, T., Okada, T. et al. Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflugers Arch - Eur J Physiol 465, 1535–1543 (2013). https://doi.org/10.1007/s00424-013-1296-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1296-y