Abstract
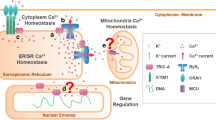
Trimeric intracellular cation-selective (TRIC) channel subtypes, namely TRIC-A and TRIC-B, are derived from distinct genes and distributed throughout the sarco/endoplasmic reticulum (SR/ER) and nuclear membranes. TRIC-A is preferentially expressed at high levels in excitable tissues, while TRIC-B is ubiquitously detected at relatively low levels in various tissues. TRIC channels are composed of ~300 amino acid residues and contain three putative membrane-spanning segments to form a bullet-shaped homo-trimeric assembly. Both native and purified recombinant TRIC subtypes form functional monovalent cation-selective channels in a lipid bilayer reconstitution system. The electrophysiological data indicate that TRIC channels behave as K+ channels under intracellular conditions, although the detailed channel characteristics remain to be investigated. The pathophysiological defects detected in knockout mice suggest that TRIC channels support SR/ER Ca2+ release mediated by ryanodine (RyR) and inositol trisphosphate receptor (IP3R) channels. For example, Tric-a-knockout mice develop hypertension resulting from vascular hypertonicity, and the mutant vascular smooth muscle cells exhibit insufficient RyR-mediated Ca2+ release for inducing hyperpolarization. Tric-b-knockout mice show respiratory failure at birth, and IP3R-mediated Ca2+ release essential for surfactant handling is impaired in the mutant alveolar epithelial cells. Moreover, double-knockout mice lacking both TRIC subtypes show embryonic heart failure, and SR Ca2+ handling is deranged in the mutant cardiomyocytes. Current evidence strongly suggests that TRIC channels mediate counter-K+ movements, in part, to facilitate physiological Ca2+ release from intracellular stores.




Similar content being viewed by others
References
Berridge MJ (2006) Calcium microdomains: organization and function. Cell Calcium 40:405–412
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529
Bezprozvanny I, Ehrlich BE (1995) The inositol 1,4,5-trisphosphate (InsP3) receptor. J Membr Biol 145:205–216
Browne LE, Jiang LH, North RA (2010) New structure enlivens interest in P2X receptors. Trends Pharmacol Sci 31:229–237
Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J (2009) MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 11:56–64
Cong Y, Ludtke SJ (2010) Single particle analysis at high resolution. Methods Enzymol 482:211–235
Coronado R, Latorre R (1982) Detection of K+ and Cl− channels from calf cardiac sarcolemma in planar lipid bilayer membranes. Nature 298:849–852
Coronado R, Miller C (1980) Decamethonium and hexamethonium block K+ channels of sarcoplasmic reticulum. Nature 288:495–497
Coronado R, Rosenberg RL, Miller C (1980) Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J Gen Physiol 76:425–446
Dani JA, Mayer ML (1995) Structure and function of glutamate and nicotinic acetylcholine receptors. Curr Opin Neurobiol 5:310–317
Fink RH, Veigel C (1996) Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle. Acta Physiol Scand 156:387–396
Fox JA (1985) Conductance and selectivity properties of a substate of the rabbit sarcoplasmic reticulum channel. Biophys J 47:573–576
Garcia AM, Miller C (1984) Channel-mediated monovalent cation fluxes in isolated sarcoplasmic reticulum vesicles. J Gen Physiol 83:819–839
Guerrero-Hernandez A, Dagnino-Acosta A, Verkhratsky A (2010) An intelligent sarco-endoplasmic reticulum Ca2+ store: release and leak channels have differential access to a concealed Ca2+ pool. Cell Calcium 48:143–149
Gillespie D, Giri J, Fill M (2009) Reinterpreting the anomalous mole fraction effect: the ryanodine receptor case study. Biophys J 97:2212–2221
Ide T, Morita T, Kawasaki T, Taguchi T, Kasai M (1991) Purification of a K+-channel protein of sarcoplasmic reticulum by assaying the channel activity in the planar lipid bilayer system. Biochim Biophys Acta 1067:213–220
Kamp F, Donoso P, Hidalgo C (1998) Changes in luminal pH caused by calcium release in sarcoplasmic reticulum vesicles. Biophys J 74:290–296
Labarca PP, Miller C (1981) A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol 61:31–38
Liu QY, Lai FA, Shen WK, Meissner G, Strauss HC (1991) Reconstitution of the solubilized cardiac sarcoplasmic reticulum potassium channel. Identification of a putative Mr approximately 80 kDa polypeptide constituent. FEBS Lett 291:13–16
McCarron JG, Olson ML, Rainbow RD, MacMillan D, Chalmers S (2007) Ins(1,4,5)P3 receptor regulation during “quantal” Ca2+ release in smooth muscle. Trends Pharmacol Sci 28:271–279
Meissner G (1983) Monovalent ion and calcium ion fluxes in sarcoplasmic reticulum. Mol Cell Biochem 55:65–82
Meissner G (1994) Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol 56:485–508
Miller C (1978) Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J Membr Biol 40:1–23
Mio K, Maruyama Y, Ogura T, Kawata M, Moriya T, Mio M, Sato C (2010) Single particle reconstruction of membrane proteins: a tool for understanding the 3D structure of disease-related macromolecules. Prog Biophys Mol Biol 103:122–130
Nishi M, Komazaki S, Kurebayashi N, Ogawa Y, Noda T, Iino M, Takeshima H (1999) Abnormal features in skeletal muscle from mice lacking mitsugumin29. J Cell Biol 147:1473–1480
Picard L, Côté K, Teijeira J, Greentree D, Rousseau E (2002) Sarcoplasmic reticulum K+ channels from human and sheep atrial cells display a specific electro-pharmacological profile. J Mol Cell Cardiol 34:1163–1172
Pitt SJ, Park KH, Nishi M, Urashima T, Aoki S, Yamazaki D, Ma J, Takeshima H, Sitsapesan R (2010) Charade of the SR K+-channel: two ion-channels, TRIC-A and TRIC-B, masquerade as a single K+-channel. Biophys J 99:417–426
Pizzo P, Lissandron V, Capitanio P, Pozzan T (2011) Ca2+ signalling in the Golgi apparatus. Cell Calcium 50:184–192
Qin F (2004) Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J 86:1488–1501
Silverio AL, Saier MH Jr (2011) Bioinformatic characterization of the trimeric intracellular cation-specific channel protein family. J Membr Biol 241:77–101
Somlyo AV, Gonzalez-Serratos H, Shuman H, McClellan G, Somlyo AP (1981) Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron probe study. J Cell Biol 90:577–594
Somlyo AV, McClellan G, Gonzalez-Serratos H, Somlyo AP (1985) Electron probe X-ray microanalysis of post-tetanic Ca2+ and Mg2+ movements across the sarcoplasmic reticulum in situ. J Biol Chem 260:6801–6807
Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M (1998) Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 17:3309–3316
Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K (2000) Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 6:11–22
Takeshima H, Shimuta M, Komazaki S, Ohmi K, Nishi M, Iino M, Miyata A, Kangawa K (1998) Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem J 331:317–322
Taylor CW, Dale P (2012) Intracellular Ca2+ channels—a growing community. Mol Cell Endocrinol 353:21–28
Tomlins B, Williams AJ (1986) Solubilisation and reconstitution of the rabbit skeletal muscle sarcoplasmic reticulum K+ channel into liposomes suitable for patch clamp studies. Pflugers Arch 407:341–347
Tomlins B, Williams AJ, Montgomery RA (1984) The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol 80:191–199
Uehara A, Yasukochi M, Imanaga I (1994) Calcium modulation of single SR potassium channel currents in heart muscle. J Mol Cell Cardiol 26:195–202
Weisleder N, Takeshima H, Ma J (2008) Immuno-proteomic approach to excitation-contraction coupling in skeletal and cardiac muscle: molecular insights revealed by the mitsugumins. Cell Calcium 43:1–8
Wellman GC, Nelson MT (2003) Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34:211–229
Xie LH, Sato D, Garfinkel A, Qu Z, Weiss JN (2008) Intracellular Ca alternans: coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J 95:3100–3110
Yamazaki D, Komazaki S, Nakanishi H, Mishima A, Nishi M, Yazawa M, Yamazaki T, Taguchi R, Takeshima H (2009) Essential role of the TRIC-B channel in Ca2+ handling of alveolar epithelial cells and in perinatal lung maturation. Development 136:2355–2361
Yamazaki D, Tabara Y, Kita S, Hanada H, Komazaki S, Naitou D, Mishima A, Nishi M, Yamamura H, Yamamoto S, Kakizawa S, Miyachi H, Yamamoto S, Miyata T, Kawano Y, Kamide K, Ogihara T, Hata A, Umemura S, Soma M, Takahashi N, Imaizumi Y, Miki T, Iwamoto T, Takeshima H (2011) TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab 14:231–241
Yazawa M, Ferrante C, Feng J, Mio K, Ogura T, Zhang M, Lin PH, Pan Z, Komazaki S, Kato K, Nishi M, Zhao X, Weisleder N, Sato C, Ma J, Takeshima H (2007) TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 448:78–82
Zeth K, Thein M (2010) Porins in prokaryotes and eukaryotes: common themes and variations. Biochem J 431:13–22
Zhang M, Yamazaki T, Yazawa M, Treves S, Nishi M, Murai M, Shibata E, Zorzato F, Takeshima H (2007) Calumin, a novel Ca2+-binding transmembrane protein on the endoplasmic reticulum. Cell Calcium 42:83–90
Zhao X, Yamazaki D, Kakizawa S, Pan Z, Takeshima H, Ma J (2011) Molecular architecture of Ca2+ signaling control in muscle and heart cells. Channels (Austin) 5:391–396
Zhao X, Yamazaki D, Park KH, Komazaki S, Tjondrokoesoemo A, Nishi M, Lin P, Hirata Y, Brotto M, Takeshima H, Ma J (2010) Ca2+ overload and sarcoplasmic reticulum instability in TRIC-A null skeletal muscle. J Biol Chem 285:37370–37376
Acknowledgments
Our TRIC channel studies were supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan, the Takeda Science Foundation, the US National Institute of Health, and the British Heart Foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Venturi, E., Sitsapesan, R., Yamazaki, D. et al. TRIC channels supporting efficient Ca2+ release from intracellular stores. Pflugers Arch - Eur J Physiol 465, 187–195 (2013). https://doi.org/10.1007/s00424-012-1197-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1197-5