Abstract
In neonatal ventricular cardiomyocytes (NVCM), decreased contractile activity stimulates sarco-endoplasmic reticulum Ca2+-ATPase2a (SERCA2a), analogous to reduced myocardial load in vivo. This study investigated in contracting NVCM the role of load-dependent RhoA-ROCK signaling in SERCA2a regulation. Contractile arrest of NVCM resulted in low peri-nuclear localized RhoA levels relative to contracting NVCM. In arrested NVCM, ROCK activity was decreased (59%) and paralleled a loss in F-actin levels. Y-27632-induced ROCK inhibition in contracting NVCM increased SERCA2a messenger RNA expression by 150%. This stimulation was transcriptional, as evident from transfections with the SERCA2a promoter. A reciprocal effect of Y-27632 treatment on the promoter activity of atrial natriuretic factor was observed. SERCA2a transcription was not altered by co-transfection of the RhoA-ROCK-dependent serum response factor (SRF) alone or in combination with myocardin. Furthermore, GATA4, another ROCK-dependent transcription factor, induced rather than repressed SERCA2a transcription. This study shows that contractile activity suppresses SERCA2a gene expression via RhoA-ROCK-dependent transcription modulation. This modulation is likely to be accomplished by a transcription factor other than SRF, myocardin, or GATA4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Workload-induced pathological cardiac hypertrophy is associated with altered expression of a subset of genes. Particular examples are the increased expression of atrial natriuretic factor (ANF) and reduced expression of sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a) [9]. The downregulation of SERCA2a affects calcium handling and contractile function and may further contribute to the progression of pathological cardiac hypertrophy. This inverse regulation of SERCA2a and ANF is observed in various in vitro studies describing the effect of enhanced contractile activity [5] or contractile arrest [3]. Moreover, in vivo unloading of the heart by left ventricular assist devices (LVAD) has also been shown to increase SERCA2a expression [2]. The transcriptional control mechanisms of SERCA2a expression remain largely unexplored.
In a recent in vitro study, we demonstrated that contractile activity inhibited SERCA2a promoter activity [31]. The mechanism of this load-induced inhibition is unclear and is the subject of the current study. One of the pathways in the load-signaling cascades in cardiac hypertrophy involves the small GTPase RhoA [20]. A principal target of the downstream RhoA effector RhoA-kinase (ROCK) is the transcription factor serum-response factor (SRF) [16]. RhoA has been implicated in the signaling cascades elicited by various hypertrophic stimuli (reviewed in [4]), including mechanical load [1], and is one of the mediators of load-induced ANF expression [20]. RhoA has been shown to be involved in actin dynamics regulation [19], but its effector ROCK can also directly phosphorylate proteins involved in excitation–contraction coupling [30]. Furthermore, RhoA-ROCK signaling can alter gene transcription through nuclear translocation of ROCK-dependent transcription factors, such as SRF [15]. SRF is a 67-kDa phosphoprotein that binds to SREs (CC (A/T)6 GG) located in SRF-responsive promoters [17], including the ANF promoter [18]. Transcriptional regulation by SRF can be modified through association with cell-specific co-factors, like the cardiac-specific factor myocardin [32]. Myocardin has recently been reported to be upregulated during heart failure [28]. Involvement of SRF in the regulation of SERCA2a transcription was indicated by a study by Zhang et al. [36], in which cardiac-specific overexpression of SRF induced cardiomyopathy with a concomitant inhibition of SERCA2a messenger RNA (mRNA) and protein expression.
Taken together, the available evidence points to a role of the RhoA-ROCK-SRF signaling pathway in mechanical load-dependent repression of SERCA2a promoter activity. The aims of the present study were to investigate the contractile activity-dependent regulation of SERCA2a by the RhoA-ROCK-SRF signaling pathway in more detail and compare this with the regulation of the hypertrophy marker ANF, using an in vitro model of contracting and quiescent neonatal ventricular cardiomyocytes (NVCM) [31].
Methods
Cell culture of neonatal rat ventricular cardiomyocytes
All animals were treated according to the national guidelines and with the permission of the Institutional Animal Care and Use Committee (IACUC) of the VU University Medical Center, The Netherlands. NVCM were isolated from 2- to 3-day-old Wistar rats (Harlan, Zwijndrecht, The Netherlands), as previously described [31]. During the experimental protocol of 3 days, isolated NVCM were allowed to contract spontaneously (CTR), or were mechanically unloaded, while largely maintaining calcium transients using the cross-bridge uncoupler 2,3-butanedione monoxime (BDM; 7.5 mM) [23] (Sigma, Zwijndrecht, The Netherlands). The role of ROCK signaling in SERCA2a mRNA expression and promoter activity was investigated using the ROCK inhibitor Y-27632 (10 μM) [15] (Tocris; Avonmouth, UK).
Immunohistochemistry
NVCM were washed in phosphate-buffered saline (PBS) at room temperature and fixed in 2% para-formaldehyde. Cells were permeabilized in ice-cold acetone/methanol (30%/70%) and were incubated with a primary rabbit anti-RhoA antibody (Santa Cruz; 1:100 in PBS+ 1% bovine serum albumin (BSA)) for 1 h and subsequently incubated with a cocktail of a secondary swine anti-rabbit antibody labeled with FITC (DAKO; 1:45 in PBS+ 1% BSA), together with rhodamine–phalloidin (1:100), to stain for F-actin filaments. Using fluorescent microscopy, cells were analyzed for actin filaments and for total RhoA protein localization.
Western blot analyses
Proteins were separated by SDS polyacrylamide gel electrophoresis. Gels were blotted onto nitrocellulose membranes (90 min at 400 mA). Membranes were stained overnight at 4°C with specific primary antibody against total α-actin (1:1,000; Sigma), against RhoA (1:1,000; Cytoskeleton, Denver, USA), or against Thr567-ezrin/radixin/moesin (ERM; 1:1,000) and total ERM (1:1,000; both Cell Signaling Technologies, Beverly, USA). The extent of phosphorylation of ERM was used as a marker of ROCK activity [19]. Protein expressions were visualized and quantified using chemiluminiscence (Amersham Biosciences, Freiburg, Germany). Equal protein loadings were confirmed by Ponceau staining.
Total RNA isolation
Total RNA was isolated from NVCM cultures, using the Tripure method according to the manufacturer’s protocol. Total RNA was quantified by A260 measurement and the A260/A280 ratio was used to check for possible contaminations.
SERCA2a mRNA expression
SERCA2a-specific primers (Invitrogen) were designed using Primer Express v2.0 to generate amplicons with a length of 75–125 bp spanning exon–exon junctions (SERCA2a; F: TTGGCTGTTATGTTGGCGC, R: AGACTCTCGGACCACCGTCA). Hypoxanthine guanine phosphoribosyltransferase (HPRT; F: ATGGGAGGCCATCACATTGT, R: ATGTAATCCAGCAGGTCAGCAA) was used as an internal control to normalize gene expressions. A total of 5 μg of total RNA was used to generate cDNA strands in a 20-μl reaction volume using the cloned AMV First Strand Synthesis Kit (Invitrogen). An equivalent of 25 ng total RNA was subsequently used in the amplification with 50 nmol/l gene-specific primers and 4 μl SYBR green master mix (Applied Biosystems) in a total volume of 8 μl, using standard cycle parameter on an Applied Biosystems model 7700.
Preparations of plasmids
The rat SERCA2a promoter fragment with 5′-ends at −6,588 bp (pLuc-S2(6.6)), at −3,263 bp (pLuc-S2(3.2)), and at −550 bp (pLuc-S2(0.5)) and 3′-ends at +550 bp (+1 is the transcription initiation site) were cloned into a promoterless pGL3-basic Luciferase vector (Promega, Madison, USA), as described previously [31]. CMV-pRL (Renilla; Promega) was used for normalization. The plasmids expressing full-length SRF (pSRF), full-length myocardin (pMyocardin), and full-length GATA4 (pGATA4) were generous gifts from Dr. R. Prywes, Dr. Parmacek, and Dr. M. van Bilsen, respectively. The SRE cis-reporter construct (pSRE) was obtained from Stratagene (La Jolla, USA).
Transient transfection assays
Luciferase reporter plasmids driven by either a minimal promoter containing five tandem repeats of SRE boxes (pLuc-SRE; 1 μg/10 cm2), driven by SERCA2a promoter fragments (250 ng/10 cm2), were transfected together with CMV-pRL plasmids (25 ng/10 cm2) in NVCM using the Fugene reagent (Roche, Almere). To investigate the role of SRF and myocardin in SERCA2a promoter regulation, pSRF and pMyocardin were co-transfected. To investigate the role of GATA4 in SERCA2a promoter regulation, pGATA4 (1 μg/10 cm2) was co-transfected. Empty pOCAT plasmid was added to the transfection mixtures to equalize total DNA during transfection. During the transfection protocol, NVCM were cultured in DMEM containing 0.2% BSA and 1% P/S. Twenty-four hours after transfection, the medium was changed and supplemented with blockers where appropriate. Two days after transfection, cells were harvested. Promoter activities were determined using the Dual Luciferase assay kit (Promega).
Statistical analysis
Data are expressed as means ± SEM and were evaluated using either a Student’s t test or an ANOVA with Bonferroni post hoc analysis. Differences were considered significant at p < 0.05.
Results
Effects of contractile arrest on actin-RhoA-ROCK signaling in cultured cardiac myocytes
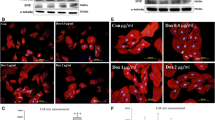
Staining of actin filaments showed that 72 h of BDM-induced contractile arrest while largely maintaining calcium transients (A. Muller et al., unpublished results; [23]) resulted in disorganization and loss of actin filaments (Fig. 1a, b), whereas Western blot analysis showed a decrease in total actin expression (Fig. 1c). This was associated with a non-significant reduction (p = 0.07) of total RhoA protein expression (2.8 ± 0.8 a.u. (mean ± SEM of three experiments) in contracting NVCM vs. 1.0 ± 0.2 a.u. (mean ± SEM of three experiments) in BDM-arrested NVCM; Fig. 2c) and a redistribution of RhoA from sarcomeric, or sarcomere-associated structures [29], to peri-nuclear regions (Fig. 2a, b). ROCK activity was reduced in these cultures, as evident from a decreased ratio of phosphorylated to total ERM relative to contracting NVCM (Fig. 2d).
Effects of contractile arrest on actin expression and distribution. a, b Actin sarcomere organization in contracting NVCM (a) and in contraction-arrested NVCM (b) as determined by immunohistochemistry. c Total actin protein expression is decreased in contraction-arrested NVCM (BDM) compared to contracting NVCM (CTR) as determined by Western blot analysis. Data are means ± SEM of at least three independent experiments. †p < 0.05. CTR contracting NVCM, BDM contraction-arrested NVCM (7.5 mM 2,3-butanedione monoxime for 72 h)
Effects of contractile arrest on RhoA-ROCK signaling. a, b Redistribution of RhoA from sarcomeric or sarcomere-associated structures in contracting NVCM (a; arrows) to peri-nuclear regions in contraction-arrested NVCM (b; arrows). c Total RhoA protein expression is reduced by 64% in contraction-arrested NVCM relative to contracting NVCM, as determined by Western blot analysis (not significant, p = 0.07). d Contractile arrest reduces phosphorylation of ezrin/radixin/moesin (ERM), a marker for ROCK activity. The ratio of phosphorylated Thr567 ERM (pERM) to total ERM was determined by Western blot analysis. Data are means ± SEM of at least three independent experiments. CTR spontaneously contracting NVCM, BDM contraction-arrested NVCM (7.5 mM 2,3-butanedione monoxime for 72 h). †p < 0.05 vs. CTR
Effects of ROCK inhibition on SERCA2a mRNA expression
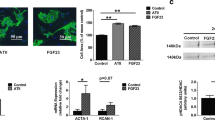
To investigate the role of ROCK in contractile activity-dependent regulation of SERCA2a mRNA expression, the level of SERCA2a mRNA was determined in RNA isolated from contracting NVCM that were either untreated or were treated with the selective ROCK-blocker Y-27632 (10 µM) [15]. Based on visual inspection, 3 days of Y-27632 treatment did not affect contraction frequency relative to spontaneously contracting NVCM, i.e., (89 ± 42 vs. 118 ± 49 beats per minute, respectively; means ± SEM of four experiments). Furthermore, rhodamine-conjugated phalloidin staining of actin filaments was comparable in control contracting NVCM and Y-27632-treated NVCM, indicating comparable contractile ability (Fig. 3a). However, effects of Y-27632 treatment on cell shortening and calcium transients cannot be excluded.
Rhodamine-conjugated phalloidin staining of actin filaments in control contracting NVCM (CTR) and Y-27632-treated NVCM (Y-27632), indicating comparable contractile ability (see also “Effects of ROCK inhibition on SERCA2a mRNA expression” in the Results section; a). Effects of ROCK inhibition on SERCA2a and ANF mRNA expression (b, c). SERCA2a mRNA level was stimulated by Y-27632-treatment (b), whereas ANF mRNA level was unchanged (c). Hypoxanthine guanine phosphoribosyltransferase (HPRT) was used as an internal control to normalize SERCA2a and ANF mRNA expression. Data are presented as means ± SEM of four independent experiments. CTR spontaneously contracting NVCM, Y-27632 contracting NVCM treated with 10 µM Y-27632. †p < 0.05 vs. CTR
Three days of exposure to Y-27632 resulted in a significant 150% increase in SERCA2a mRNA expression (Fig. 3b) without affecting ANF mRNA expression (Fig. 3c). The increase in SERCA2a mRNA levels in BDM-treated cultures was similar to the increase in Y-27632-treated cultures (2.5 ± 0.1 (n = 5) and 2.5 ± 0.6 (n = 4), respectively; means ± SEM).
ROCK signaling and SERCA2a transcription regulation
Next, we investigated whether transcriptional mechanisms underlie the ROCK-induced changes in SERCA2a mRNA expression. Contracting NVCM were transiently transfected with luciferase-reporter plasmids driven by SERCA2a promoter fragments of different lengths (0.55, 3.3, and 6.6 kb, respectively). Treatment with Y-27632 did not affect the activity of the 0.55 and 3.3 kb SERCA2a promoter fragment, but increased the transcriptional activity of the 6.6-kb SERCA2a promoter fragment by 80% (Fig. 4a, b). Treatment with Y-27632 significantly decreased ANF promoter activity (−41%; Fig. 4d).
Effects of ROCK inhibition by Y-27632 on SERCA2a and ANF promoter activity. Contracting NVCM were transfected with promoter-luciferase (Luc) reporter plasmids driven by 0.55 kb (a), 3.3 kb (b), or 6.6 kb (c) of the SERCA2a promoter sequence or 638 bp of the ANF promoter sequence (d). The CMV-Renilla (Ren) plasmid was co-transfected for normalization of the luciferase expression. Y-27632 treatment did not affect the activity of the 0.55 or 3.3 kb SERCA2a promoter fragment (a, b), but stimulated the activity of the 6.6-kb SERCA2a promoter fragment (c), whereas ANF promoter activity was repressed (d). Data are presented as means ± SEM of six independent experiments. CTR spontaneously contracting NVCM, Y-27632 contracting NVCM treated with 10 µM Y-27632 (treatment did not affect contractile activity). *p < 0.001 vs. CTR
Effects of contractile arrest and ROCK inhibition on SRF activity
To monitor the changes in activity of the ROCK-dependent transcription factor SRF induced by contractile arrest and ROCK inhibition, transfection experiments were performed in NVCM, using a luciferase reporter construct driven by a promoter containing multiple serum response elements (pLuc-SRE). In these experiments, stimulation of the activity of the SRE-containing promoter by an SRF expression plasmid (pSRF) was used as a positive control (2.2-fold increase; Fig. 5a). Spontaneously contracting NVCM, cultured with or without the ROCK inhibitor Y-27632, and contraction-arrested NVCM were transiently transfected with pLuc-SRE. The activity of the SRE-containing promoter was lower in spontaneously contracting NVCM treated with the ROCK inhibitor Y-27632 (−25%) and in contraction-arrested NVCM (−75%), compared to untreated NVCM (Fig. 5a). This indicates that ROCK is involved in contractile activity-mediated SRF activation.
SRE activity is decreased by contractile arrest and ROCK inhibition and stimulated by SRF (a). Direct effects of SRF alone or in combination with its co-factor myocardin on SERCA2a promoter activity were not found. GATA4 increased SERCA2a promoter activity (b). Data are presented as means ± SEM of at least five independent experiments. CTR contracting NVCM, Y-27632 contracting NVCM treated with 10 µM Y-27632, BDM contraction-arrested NVCM (7.5 mM 2,3-butanedione monoxime for 72 h); ***p < 0.05 vs. CTR, **p < 0.001 vs. CTR, *p < 0.001 vs. CTR
SERCA2a transcription regulation by SRF
The role of SRF in regulating SERCA2a gene transcription is unexplored. To investigate whether SRF is a candidate mediator of the ROCK-dependent decrease of SERCA2a promoter activity, co-transfection studies were performed in NVCM using the pLuc-S2(6.6) reporter construct and an SRF expression plasmid (pSRF). pSRF was transfected at an input of 100 pg plasmid/10 cm2 [22, 37]. Transfection of pSRF did not result in alteration of SERCA2a promoter activity. Myocardin was subsequently tested as a potential co-factor required for the repression of promoter activity. However, co-transfection of a myocardin expression vector (pMyocardin; 100 pg/10 cm2) was without effect (Fig. 5b).
The activity of the transcription factor GATA4 is implicated in hypertrophy and is also increased by RhoA-ROCK signaling. GATA4 is therefore a potential mediator of the ROCK-dependent decrease of SERCA2a promoter activity. However, the data in Fig. 5b show that GATA4 is an activator of SERCA2a promoter activity rather than a repressor.
Discussion
Contractile activity of NVCM has previously been demonstrated to decrease SERCA2a mRNA expression. The present study provides evidence for an inhibitory role of contraction-induced RhoA-ROCK signaling in the regulation of SERCA2a gene transcription and mRNA expression.
Development of maladaptive myocardial hypertrophy as a result of hemodynamic overload is associated with upregulation of ANF mRNA expression and downregulation of SERCA2a mRNA expression [9]. Maladaptive myocardial hypertrophy and unfavorable LV remodeling can result from enhanced RhoA signaling [14, 24], which is associated with altered myofibrillar organization [12, 33]. In a variety of experiments using pro-hypertrophic stimuli such as lysophosphatidic acid [11], phenylephrine [34], and mechanical stress [20], enhanced RhoA signaling has been demonstrated to result in upregulation of ANF mRNA expression.
Although the role of RhoA signaling in the regulation of ANF mRNA expression and transcription is well established [12, 27], its role in SERCA2a promoter regulation remains to be elucidated. In contraction-arrested NVCM, SERCA2a mRNA expression was recently demonstrated to depend on the upregulation of SERCA2a promoter activity by a synergistic action of NFATc4 and MEF2c, the downstream transcription factors of the calcium-dependent calcineurin and CAMK-II pathways, respectively [31]. In this model, contractile activity suppressed SERCA2a mRNA expression by a mechanism that was apparently independent of the calcineurin signaling pathway since nuclear translocation of NFAT was similar in spontaneously contracting and arrested NVCM. It remained unclear, however, whether altered SERCA2a promoter activities underlie the observed load-dependent changes in mRNA expression levels, and which signaling pathways are involved. The current study shows RhoA-ROCK signaling to be involved in the downregulation of SERCA2a promoter activity as a result of contractile activity of NVCM. In contraction-arrested NVCM, redistribution of RhoA to peri-nuclear levels paralleled a loss in F-actin structures and coincided with low activity of the RhoA-target protein ROCK. Inhibition of ROCK in contracting NVCM increased SERCA2a mRNA at the level of gene transcription, but the RhoA-ROCK-dependent transcription factors SRF, myocardin, and GATA4 appeared not to be involved in the repression of SERCA2a transcription. The contractile activity-dependent decrease of SERCA2a transcription is likely to be accomplished by an as yet unknown RhoA-ROCK-dependent transcription factor.
Effects of contractile arrest on RhoA-ROCK signaling
Following 3 days of contractile arrest, the cultured NVCM exhibited disorganization of the cytoskeleton with a concomitant decreased expression of actin, as evident from the rhodamine–phalloidin staining of the actin filaments and from Western blot analysis, respectively. Since RhoA-ROCK signaling is closely linked to actin organization, the previously observed contractile arrest-induced increase in SERCA2a mRNA expression [31] could have resulted from suppression of RhoA-ROCK signaling because of cytoskeletal disorganization. The decrease in total RhoA expression, its altered distribution to more peri-nuclear regions, and the observed decreased activity of its substrate, ROCK, strongly suggest that unloading results in impaired RhoA-ROCK signaling.
Role of ROCK signaling SERCA2a transcription regulation
Selective ROCK inhibition by Y-27632 in contracting NVCM resulted in upregulation of SERCA2a promoter activity, which was accompanied by downregulation of ANF promoter activity. This ability of ROCK to induce reciprocal changes in SERCA2a and ANF gene transcription confirms an earlier report which showed that decreased Rho kinase activity blunts pathological myocardial hypertrophy in a model of hypertension-induced heart failure [25].
Examination of various lengths of the SERCA2a promoter revealed that only the activity of the 6.6-kb fragment, not of the 0.55- or 3.3-kb fragment, was increased by ROCK inhibition. This suggests that RhoA-ROCK-sensitive regions are located in the far-upstream regions of the promoter. The increased activity of the 6.6-kb SERCA2a promoter in ROCK-inhibited NVCM corresponded with an increased SERCA2a mRNA expression, suggesting that the far-upstream region of the SERCA2a promoter is of physiological significance. The previously reported increase of SERCA2a promoter activity by the Ca2+ signaling-dependent transcription factors NFAT and MEF2 [31] also involved the far-upstream region of the SERCA2a promoter. The present study suggests that the effect of these stimulatory transcription factors is counteracted by inhibitory mechanical load-induced RhoA-ROCK-dependent transcription factors. Although SERCA2a promoter activity was modulated to a larger extent by NFAT and MEF compared to the ROCK-dependent regulation (a 3.5-fold increase induced by NFAT/MEF2 compared to a 1.75-fold increase by ROCK inhibition), we cannot draw conclusions concerning the relative importance of these pathways due to the differences in experimental setup. In the NFAT/MEF2 transfection experiments, co-transfection with NFAT/MEF expression plasmids provided saturating levels of these transcription factors, while the effect of ROCK inhibition by Y-27632 treatment on SERCA2a promoter activity was dependent on endogenous levels of transcription factors, which may have been limiting.
Analysis of the expression of an SRE-driven reporter gene in contracting NVCM showed that SRF is a contraction- and ROCK-sensitive transcription factor. The observed large difference in SRF activity between Y-27632-treated cells and BDM-treated cells (reduction of 25% and 75%, respectively, as compared to control cultures) may result from activation of load-dependent pathways that activate SRF, other than the RhoA-ROCK pathway, that remain active in the still contracting Y-27632-treated cells but are abolished in the contraction-arrested BDM-treated cells. A candidate is the load-dependent TGFβ-activated kinase pathway, which has been shown to stimulate SRE activity [35].
The present study shows that SERCA2a promoter activity is not repressed by SRF alone, or in combination with its co-factor myocardin, suggesting that another RhoA-ROCK-dependent transcription factor is involved in the observed contractile activity-dependent repression of SERCA2a expression.
The transcription factor GATA4 was considered to be a likely candidate. It has been described that RhoA-ROCK signaling participates in GATA4-dependent effects of hypertrophic neurohumoral factors via the ERK [34] or p38-MAPKK pathway [6] in NVCM. Furthermore, it was shown that GATA4 is a mediator of stretch-induced cardiomyocyte hypertrophy [21]. Taken together, these data indicate that GATA4 is a potential mediator of RhoA-ROCK-induced decrease of SERCA2a transcription. This study shows that GATA4 stimulated SERCA2a promoter activity, which is opposite to the expected inhibition if GATA4 were the downstream transcription factor of the ROCK signaling cascade regulating SERCA2a expression. These results appear to exclude not only SRF, but also GATA4 as ROCK-dependent transcription factors mediating the load-dependent inhibition of SERCA2a expression in this model.
Study limitations
The use of BDM as a cross-bridge uncoupler in our experimental setup allows the dissection of calcium-dependent and load-dependent effects of contractile activity, which is not possible in in vivo models of cardiac hypertrophy. A restriction of the use of the chemical phosphatase BDM is that it may influence the activity of proteins other than myosin ATPase, like the L-type calcium channel and gap junction channels [7, 8, 26]. However, these effects were observed at higher concentrations of BDM (between 15-50 mM) than those required to inhibit contractile activity in the present study (7.5 mM). Although calcium transients are largely maintained in the presence of 7.5 mM BDM (A. Muller et al, unpublished results; [23]), it cannot be excluded that changes in calcium transients influenced the effects observed upon BDM-treatment and therefore cannot be fully ascribed to changes in mechanical load. Similarly, changes in calcium transients due to Y-27632 treatment cannot be excluded.
A limitation of the experimental setup used in this in vitro study is that a condition of complete absence of mechanical load is compared to a condition of mechanical load imposed by spontaneous contractions, whereas cardiac hypertrophy in vivo develops in response to a sustained increase in mechanical load of contracting myocytes. Despite this limitation of the model of contractile arrest induced by BDM treatment, the model may be used to elucidate basic mechanical load-dependent regulatory mechanisms. Cadre et al. [5] showed that stretching of NVCM induced a decrease of SERCA2a expression, which indicates that there is a resemblance between mechanical load resulting from spontaneous contractions and the mechanical load resulting from mechanical deformation by stretch. Furthermore, a recent study by Jacot et al. [13] showed that NVCM that were grown on substrates with variable stiffness, a very stiff substrate, resulted in decreased SERCA2a mRNA expression, which was suggested to involve RhoA-ROCK signaling.
An in vivo condition that may resemble to some extent our in vitro unloading approach is the use of human LVAD. During end-stage heart failure, patients receive LVAD to reduce hemodynamic overload in order to bridge the period preceding cardiac transplantation. LVAD treatment improved cardiac function by inducing a process called reverse remodeling, with improved cardiac relaxation and increased expression levels of calcium handling proteins including SERCA2a [10].
References
Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, Kadowaki T, Yazaki Y (1999) Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ Res 84:458–466
Barbone A, Holmes JW, Heerdt PM, The AH, Naka Y, Joshi N, Daines M, Marks AR, Oz MC, Burkhoff D (2001) Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: a primary role of mechanical unloading underlying reverse remodeling. Circulation 104:670–675
Bassani JW, Qi M, Samarel AM, Bers DM (1994) Contractile arrest increases sarcoplasmic reticulum calcium uptake and SERCA2 gene expression in cultured neonatal rat heart cells. Circ Res 74:991–997
Brown JH, Del Re DP, Sussman MA (2006) The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res 98:730–742
Cadre BM, Qi M, Eble DM, Shannon TR, Bers DM, Samarel AM (1998) Cyclic stretch down-regulates calcium transporter gene expression in neonatal rat ventricular myocytes. J Mol Cell Cardiol 30:2247–2259
Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M (2001) Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev 15:2702–2719
Duthe F, Dupont E, Verrecchia F, Plaisance I, Severs NJ, Sarrouilhe D, Herve JC (2000) Dephosphorylation agents depress gap junctional communication between rat cardiac cells without modifying the Connexin43 phosphorylation degree. Gen Physiol Biophys 19:441–449
Ferreira G, Artigas P, Pizarro Gustavo Brum G (1997) Butanedione monoxime promotes voltage-dependent inactivation of-type calcium channels in heart. Effects on gating currents. J Mol Cell Cardiol 29:777–787
Frey N, Olson EN (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65:45–79
Heerdt PM, Holmes JW, Cai B, Barbone A, Madigan JD, Reiken S, Lee DL, Oz MC, Marks AR, Burkhoff D (2000) Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation 102:2713–2719
Hilal-Dandan R, Means CK, Gustafsson AB, Morissette MR, Adams JW, Brunton LL, Heller Brown J (2004) Lysophosphatidic acid induces hypertrophy of neonatal cardiac myocytes via activation of Gi and Rho. J Mol Cell Cardiol 36:481–493
Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH (1998) The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of RhoKinase. J Biol Chem 273:7725–7730
Jacot JG, McCullock AD, Omens JH (2008) Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95:3479–3487
Kobayashi N, Horinaka S, Mita Si, Nakano S, Honda T, Yoshida K, Kobayashi T, Matsuoka H (2002) Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc Res 55:757–767
Liu HW, Halayko AJ, Fernandes DJ, Harmon GS, McCauley JA, Kocieniewski P, McConville J, Fu Y, Forsythe SM, Kogut P, Bellam S, Dowell M, Churchill J, Lesso H, Kassiri K, Mitchell RW, Hershenson MB, Camoretti-Mercado B, Solway J (2003) The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol 29:39–47
Loirand G, Guerin P, Pacaud P (2006) Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98:322–334
Miano JM (2003) Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 35:577–593
Morissette MR, Sah VP, Glembotski CC, Brown JH (2000) The Rho effector, PKN, regulates ANF gene transcription in cardiomyocytes through a serum response element. Am J Physiol Heart Circ Physiol 278:H1769–H1774
van Nieuw Amerongen GP, van Hinsbergh VWM (2001) Cytoskeletal effects of Rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb Vasc Biol 21:300–311
Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, Baker KM (2005) PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol 202:536–553
Pikkarainen S, Tokola H, Majalahti-Palviainen T, Kerkela R, Hautala N, Bhalla SS, Charron F, Nemer M, Vuolteenaho O, Ruskoaho H (2003) GATA-4 is a nuclear mediator of mechanical stretch-activated hypertrophic program. J Biol Chem 278:23807–23816
Prywes R, Zhu H (1992) In vitro squelching of activated transcription by serum response factor: evidence for a common coactivator used by multiple transcriptional activators. Nucleic Acids Res 20:513–520
Qi M, Puglisi JL, Byron KL, Ojamaa K, Klein I, Bers DM, Samarel AM (1997) Myosin heavy chain gene expression in neonatal rat heart cells: effects of [Ca2+]i and contractile activity. Am J Physiol Cell Physiol 273:C394–C403
Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW, Ross J Jr, Chien KR, Brown JH (1999) Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest 103:1627–1634
Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, Makino N (2003) Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol 35:59–70
Stapleton MT, Fuchsbauer CM, Allshire AP (1998) BDM drives protein dephosphorylation and inhibits adenine nucleotide exchange in cardiomyocytes. Am J Physiol Heart Circ Physiol 275:H1260–H1266
Thorburn J, Xu SC, Thorburn A (1997) MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J 16:1888–1900
Torrado M, Lopez E, Centeno A, Medrano C, Castro-Beiras A, Mikhailov AT (2003) Myocardin mRNA is augmented in the failing myocardium: expression profiling in the porcine model and human dilated cardiomyopathy. J Mol Med 81:566–577
Torsoni AS, Marin TM, Velloso LA, Franchini KG (2005) RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. AJP Heart Circ Physiol 289:H1488–96
Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ (2005) Functional effects of rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ Res 96:740–747
Vlasblom R, Muller A, Musters RJP, Zuidwijk MJ, van Hardeveld C, Paulus WJ, Simonides WS (2004) Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc Res 63:537–544
Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN (2004) Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428:185–189
Witteck A, Yao Y, Fechir M, Forstermann U, Kleinert H (2003) Rho protein-mediated changes in the structure of the actin cytoskeleton regulate human inducible NO synthase gene expression. Exp Cell Res 287:106–115
Yanazume T, Hasegawa K, Wada H, Morimoto T, Abe M, Kawamura T, Sasayama S (2002) Rho/ROCK pathway contributes to the activation of extracellular signal-regulated kinase/GATA-4 during myocardial cell hypertrophy. J Biol Chem 277:8618–8625
Zhang D, GaussinV Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med 6:556–563
Zhang X, Azhar G, Chai J, Sheridan P, Nagano K, Brown T, Yang J, Khrapko K, Borras AM, Lawitts J, Misra RP, Wei JY (2001) Cardiomyopathy in transgenic mice with cardiac-specific overexpression of serum response factor. Am J Physiol Heart Circ Physiol 280:H1782–H1792
Zhu H, Joliot V, Prywes R (1994) Role of transcription factor TFIIF in serum response factor-activated transcription. J Biol Chem 269:3489–3497
Acknowledgment
GPvNA was supported by The Netherlands Heart Foundation (The Hague, grant 2003T032).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Vlasblom, R., Muller, A., Beckers, C.M.L. et al. RhoA-ROCK signaling is involved in contraction-mediated inhibition of SERCA2a expression in cardiomyocytes. Pflugers Arch - Eur J Physiol 458, 785–793 (2009). https://doi.org/10.1007/s00424-009-0659-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0659-x