Abstract
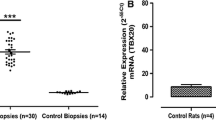
The implication of myocardin and homeodomain only protein (HOP) in combinatorial molecular pathways that guide heart development and cardio-specific gene expression has recently been reported. However, expression of these genes in the failing heart has not yet been investigated. This study was designed to elaborate a molecular profile of myocardin and HOP expression in the failing ventricular myocardium through the use of both explanted human heart samples and heart biopsies from neonatal piglets with doxorubicin-induced cardiomyopathy (Dox-CM). Myocardin and HOP mRNA levels were estimated by both northern blot hybridization and semiquantitative RT-PCR in human ventricular preparations in end-stage failure due to dilated cardiomyopathy (DCM), as well as in nonfailing donor hearts. Similar experiments were performed with ventricular samples from normal and Dox-treated neonatal piglets. The gene expression of brain natriuretic peptide (BNP) was used as a molecular marker of myocardial damage and failure. The study revealed the following novel findings: (1) myocardin transcripts are detected in neonatal human and pig hearts at lower levels than in mature cardiac tissues, (2) the myocardin transcript pool is significantly augmented in the failing human and porcine myocardium as compared to that in nonfailing heart samples, (3) in the failing human myocardium, increased levels of myocardin mRNA are associated with a diminished HOP transcript content, and (4) the inverse proportion in cardiac myocardin/HOP mRNA pools observed in explanted human hearts is also traceable in normal human heart and aorta. A possible dual consequence of increased myocardin and decreased HOP expression levels on serum response factor-dependent cardiac-specific expression in the normal heart and at heart failure is discussed. Therefore, increased abundance of the myocardin mRNA pool is judged to be a novel CM-related feature which, alone or in association with decreased HOP transcript levels, can be responsible for dysregulation of myocardin-mediated gene expression in failing myocardium.






Similar content being viewed by others
Abbreviations
- Dox-CM :
-
Doxorubicin-induced cardiomyopathy
- RT-PCR :
-
Reverse transcriptase-polymerase chain reaction
- BNP :
-
Brain natriuretic peptide
- ANP :
-
Atrial natriuretic peptide
- DCM :
-
Dilated cardiomyopathy
- LV :
-
Left ventricle
- RV :
-
Right ventricle
- LA :
-
Left atrium
- RA :
-
Right atrium
- AP :
-
Apex of the heart
- NIS :
-
Normal isotonic saline
- HOP :
-
Homeodomain only protein
- SRF :
-
Serum response factor
- SMC :
-
Smooth muscle cells
- UTR :
-
Untranslated region
- FW :
-
Free-wall
- MLC2v :
-
Myosin light chain 2 ventricular form
- SM-A :
-
Smooth muscle alpha actin
- Alpha-MHC :
-
Alpha myosin heavy chain
References
Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN (2001) Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105:851–862
Chen J, Kitchen CM, Streb JW, Miano JM (2002) Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34:1345–1356
Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS (2003) Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 23:2425–2437
Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK (2003) Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 92:856–865
Kumar MS, Owens GK (2003) Combinatorial control of smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol 23:737–747
Chien KR, Olson EN (2002) Converging pathways and principles in heart development and disease: CV@CSH. Cell 110:153–162
Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN (2002) Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci U S A 99:14855–14860
Zhang X, Azhar G, Chai J, Sheridan P, Nagano K, Brown T, Yang J, Khrapko K, Borras AM, Lawitts J, Misra RP, Wei JY (2001) Cardiomyopathy in transgenic mice with cardiac-specific overexpression of serum response factor. Am J Physiol Heart Circ Physiol 280:H1782–1792
Zhang X, Chai J, Azhar G, Sheridan P, Borras AM, Furr MC, Khrapko K, Lawitts J, Misra RP, Wei JY (2001) Early postnatal cardiac changes and premature death in transgenic mice overexpressing a mutant form of serum response factor. J Biol Chem 276:40033–40040
Chai J, Tarnawski AS (2002) Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol 53:147–157
Davis FJ, Gupta M, Pogwizd SM, Bacha E, Jeevanandam V, Gupta MP (2002) Increased expression of alternatively spliced dominant-negative isoform of SRF in human failing hearts. Am J Physiol Heart Circ Physiol 282:H1521–1533
Shin CH, Liu ZP, Passier R, Zhang CL, Wang DZ, Harris TM, Yamagishi H, Richardson JA, Childs G, Olson EN (2002) Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell 110:725–735
Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC, Epstein JA (2002) Hop is an unusual homeobox gene that modulates cardiac development. Cell 110:713–723
(1997) World Medical Association. World Medical Association declaration of Helsinki. Cardiovasc Res 35:2–3
Ahmed F, Torrado M, Johnson E, Morrison J, Tomarev SI (2001) Changes in mRNA levels of the Myoc/Tigr gene in the rat eye after experimental elevation of intraocular pressure or optic nerve transection. Invest Ophthalmol Vis Sci 42:3165–3172
Torrado M, Lopez E, Centeno A, Castro-Beiras A, Mikhailov AT (2003) The asymmetric distribution of the CARP mRNA in the left and right ventricle myocardium of the piglet heart is altered in a postnatal model of doxorubucin-induced heart failure. Eur J Heart Fail Suppl 2:10
Sussman MA, Hamm-Alvarez SF, Vilalta PM, Welch S, Kedes L (1997) Involvement of phosphorylation in doxorubicin-mediated myofibril degeneration. An immunofluorescence microscopy analysis. Circ Res 80:52–61
Kremer LC, Tiel-van Buul MM, Ubbink MC, Offringa M, Ottenkamp J, Olmos RV, Voute PA (1999) Indium-111-antimyosin scintigraphy in the early detection of heart damage after anthracycline therapy in children. J Clin Oncol 17:1208
Wang Z, Wang DZ, Pipes GC, Olson EN (2003) Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A 100:7129–7134
Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR (1997) Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest 100:2315–2324
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104:2923–2931
Haase D, Lehmann MH, Korner MM, Korfer R, Sigusch HH, Figulla HR (2002) Identification and validation of selective upregulation of ventricular myosin light chain type 2 mRNA in idiopathic dilated cardiomyopathy. Eur J Heart Fail 4:23–31
Adachi S, Ito H, Tamamori M, Tanaka M, Marumo F, Hiroe M (1998) Skeletal and smooth muscle alpha-actin mRNA in endomyocardial biopsy samples of dilated cardiomyopathy patients. Life Sci 63:1779–1791
Miyata S, Minobe W, Bristow MR, Leinwand LA (2000) Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86:386–390
Acknowledgements
This work was supported by a long-time grant (SAF2001-0910) from the Spanish Ministry of Science and Technology and by infrastructure funding (2002) from the Autonomic Government of Galicia (Spain). We thank Dr. Marisa Crespo and Dr. J. Muñiz for their help in sampling and characterization of human heart tissues. We are grateful to Dr. Esperanza Cerdán for welcoming our northern blot hybridization experiments in her laboratory. We wish to thank two anonymous reviewers for constructive comments on the first version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torrado, M., López, E., Centeno, A. et al. Myocardin mRNA is augmented in the failing myocardium: expression profiling in the porcine model and human dilated cardiomyopathy. J Mol Med 81, 566–577 (2003). https://doi.org/10.1007/s00109-003-0470-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-003-0470-7