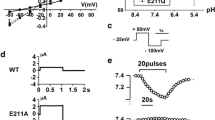
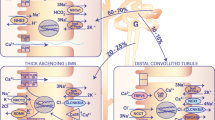
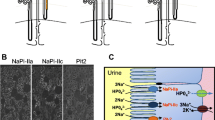
Abstract
The CLC gene family encodes Cl− channels or Cl−/H+ exchangers. While our understanding of their structure–function relationship has greatly benefited from the crystal structure of bacterial homologues, human inherited diseases and knock-out mice were crucial in deciphering their physiological roles. Several vesicular CLC Cl−/H+ exchangers are expressed in the proximal tubule (PT). ClC-5 mutations cause Dent’s disease which is associated with low molecular weight proteinuria and kidney stones. ClC-5 knock-out mice revealed impaired endocytosis as the primary defect in Dent’s disease. It extends to receptor-mediated and fluid-phase endocytosis and entails changes in calciotropic hormones that result in kidney stones. No renal functions could be assigned so far to ClC-3 and ClC-4, which are also expressed in PTs. Loss of ClC-7 or its β-subunit Ostm1 entails lysosomal storage in the PT, in addition to the neuronal lysosomal storage and osteopetrosis that are the hallmarks of ClC-7/Ostm1 loss in mice and men.




Similar content being viewed by others
References
Jentsch TJ, Steinmeyer K, Schwarz G (1990) Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature 348:510–514
Miller C (1982) Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci 299:401–411
Miller C, White MM (1984) Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A 81:2772–2775
Bauer CK, Steinmeyer K, Schwarz JR, Jentsch TJ (1991) Completely functional double-barreled chloride channel expressed from a single Torpedo cDNA. Proc Natl Acad Sci U S A 88:11052–11056
Ludewig U, Pusch M, Jentsch TJ (1996) Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature 383:340–343
Middleton RE, Pheasant DJ, Miller C (1996) Homodimeric architecture of a ClC-type chloride ion channel. Nature 383:337–340
Weinreich F, Jentsch TJ (2001) Pores formed by single subunits in mixed dimers of different CLC chloride channels. J Biol Chem 276:2347–2353
Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415:287–294
Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ (1995) Gating of the voltage-dependent chloride channel ClC-0 by the permeant anion. Nature 373:527–531
Dutzler R, Campbell EB, MacKinnon R (2003) Gating the selectivity filter in ClC chloride channels. Science 300:108–112
Accardi A, Miller C (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427:803–807
Picollo A, Pusch M (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436:420–423
Scheel O, Zdebik A, Lourdel S, Jentsch TJ (2005) Voltage-dependent electrogenic chloride proton exchange by endosomal CLC proteins. Nature 436:424–427
Lisal J, Maduke M (2008) The ClC-0 chloride channel is a ‘broken’ Cl−/H+ antiporter. Nat Struct Mol Biol 15:805–810
Lorenz C, Pusch M, Jentsch TJ (1996) Heteromultimeric CLC chloride channels with novel properties. Proc Natl Acad Sci U S A 93:13362–13366
Suzuki T, Rai T, Hayama A, Sohara E, Suda S, Itoh T, Sasaki S, Uchida S (2006) Intracellular localization of ClC chloride channels and their ability to form hetero-oligomers. J Cell Physiol 206:792–798
Mohammad-Panah R, Harrison R, Dhani S, Ackerley C, Huan LJ, Wang Y, Bear CE (2003) The chloride channel ClC-4 contributes to endosomal acidification and trafficking. J Biol Chem 278:29267–29277
Maritzen T, Rickheit G, Schmitt A, Jentsch TJ (2006) Kidney-specific upregulation of vitamin D3 target genes in ClC-5 KO mice. Kidney Int 70:79–87
Meyer S, Dutzler R (2006) Crystal structure of the cytoplasmic domain of the chloride channel ClC-0. Structure 14:299–307
Wellhauser L, Kuo HH, Stratford FL, Ramjeesingh M, Huan LJ, Luong W, Li C, Deber CM, Bear CE (2006) Nucleotides bind to the C-terminus of ClC-5. Biochem J 398:289–294
Markovic S, Dutzler R (2007) The structure of the cytoplasmic domain of the chloride channel ClC-Ka reveals a conserved interaction interface. Structure 15:715–725
Meyer S, Savaresi S, Forster IC, Dutzler R (2007) Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat Struct Mol Biol 14:60–67
Bennetts B, Rychkov GY, Ng HL, Morton CJ, Stapleton D, Parker MW, Cromer BA (2005) Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J Biol Chem 280:32452–32458
Dhani SU, Kim Chiaw P, Huan LJ, Bear CE (2008) ATP depletion inhibits the endocytosis of ClC-2. J Cell Physiol 214:273–280
Zifarelli G, Pusch M (2008) The muscle chloride channel ClC-1 is not directly regulated by intracellular ATP. J Gen Physiol 131:109–116
Fong P, Rehfeldt A, Jentsch TJ (1998) Determinants of slow gating in ClC-0, the voltage-gated chloride channel of Torpedo marmorata. Am J Physiol 274:C966–C973
Estévez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ (2004) Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol 557:363–378
Schwappach B, Stobrawa S, Hechenberger M, Steinmeyer K, Jentsch TJ (1998) Golgi localization and functionally important domains in the NH2 and COOH terminus of the yeast CLC putative chloride channel Gef1p. J Biol Chem 273:15110–15118
Estévez R, Boettger T, Stein V, Birkenhäger R, Otto M, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl− channel β-subunit crucial for renal Cl− reabsorption and inner ear K+-secretion. Nature 414:558–561
Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC (2006) ClC-7 requires Ostm1 as a β-subunit to support bone resorption and lysosomal function. Nature 440:220–223
Scholl U, Hebeisen S, Janssen AG, Müller-Newen G, Alekov A, Fahlke C (2006) Barttin modulates trafficking and function of ClC-K channels. Proc Natl Acad Sci U S A 103:11411–11416
Steinmeyer K, Klocke R, Ortland C, Gronemeier M, Jockusch H, Gründer S, Jentsch TJ (1991) Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature 354:304–308
Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ (1992) The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 257:797–800
Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP (1997) Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet 17:171–178
Matsumura Y, Uchida S, Kondo Y, Miyazaki H, Ko SB, Hayama A, Morimoto T, Liu W, Arisawa M, Sasaki S, Marumo F (1999) Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet 21:95–98
Bösl MR, Stein V, Hübner C, Zdebik AA, Jordt SE, Mukhophadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ (2001) Male germ cells and photoreceptors, both depending on close cell–cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J 20:1289–1299
Zdebik AA, Cuffe J, Bertog M, Korbmacher C, Jentsch TJ (2004) Additional disruption of the ClC-2 Cl− channel does not exacerbate the cystic fibrosis phenotype of CFTR mouse models. J Biol Chem 279:22276–2226783
Jentsch TJ (2008) CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43:3–36
Birkenhäger R, Otto E, Schürmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NVAM, Antignac C, Sudbrack R, Kispert A, Hildebrandt F (2001) Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29:310–314
Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hübner CA, Jentsch TJ (2007) Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 27:6581–6589
Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bösl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29:185–196
Jentsch TJ (2007) Chloride and the endosomal–lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol 578:633–640
Li X, Shimada K, Showalter LA, Weinman SA (2000) Biophysical properties of ClC-3 differentiate it from swelling-activated chloride channels in Chinese hamster ovary-K1 cells. J Biol Chem 275:35994–35998
Graves AR, Curran PK, Smith CL, Mindell JA (2008) The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453:788–792
Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215
Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poët M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ (2005) Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J 24:1079–1091
Günther W, Lüchow A, Cluzeaud F, Vandewalle A, Jentsch TJ (1998) ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci U S A 95:8075–8080
Hara-Chikuma M, Yang B, Sonawane ND, Sasaki S, Uchida S, Verkman AS (2005) ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J Biol Chem 280:1241–1247
Günther W, Piwon N, Jentsch TJ (2003) The ClC-5 chloride channel knock-out mouse—an animal model for Dent’s disease. Pflügers Arch 445:456–462
Hara-Chikuma M, Wang Y, Guggino SE, Guggino WB, Verkman AS (2005) Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem Biophys Res Commun 329:941–946
De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442:939–942
Fisher SE, Black GC, Lloyd SE, Hatchwell E, Wrong O, Thakker RV, Craig IW (1994) Isolation and partial characterization of a chloride channel gene which is expressed in kidney and is a candidate for Dent’s disease (an X-linked hereditary nephrolithiasis). Hum Mol Genet 3:2053–2059
Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, Bolino A, Devoto M, Goodyer P, Rigden SP, Wrong O, Jentsch TJ, Craig IW, Thakker RV (1996) A common molecular basis for three inherited kidney stone diseases. Nature 379:445–449
Dickerson LW, Bonthius DJ, Schutte BC, Yang B, Barna TJ, Bailey MC, Nehrke K, Williamson RA, Lamb FS (2002) Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res 958:227–250
Yoshikawa M, Uchida S, Ezaki J, Rai T, Hayama A, Kobayashi K, Kida Y, Noda M, Koike M, Uchiyama Y, Marumo F, Kominami E, Sasaki S (2002) CLC-3 deficiency leads to phenotypes similar to human neuronal ceroid lipofuscinosis. Genes Cells 7:597–605
Poët M, Kornak U, Schweizer M, Zdebik AA, Scheel O, Hoelter S, Wurst W, Schmitt A, Fuhrmann JC, Planells-Cases R, Mole SE, Hübner CA, Jentsch TJ (2006) Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc Natl Acad Sci U S A 103:13854–13859
Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ (1994) Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci U S A 91:6943–6947
Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F (1995) Localization and functional characterization of rat kidney-specific chloride channel, ClC-K1. J Clin Invest 95:104–113
Vandewalle A, Cluzeaud F, Bens M, Kieferle S, Steinmeyer K, Jentsch TJ (1997) Localization and induction by dehydration of ClC-K chloride channels in the rat kidney. Am J Physiol 272:F678–F688
Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F (2001) Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12:1327–1334
Thiemann A, Gründer S, Pusch M, Jentsch TJ (1992) A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356:57–60
Obermüller N, Gretz N, Kriz W, Reilly RF, Witzgall R (1998) The swelling-activated chloride channel ClC-2, the chloride channel ClC-3, and ClC-5, a chloride channel mutated in kidney stone disease, are expressed in distinct subpopulations of renal epithelial cells. J Clin Invest 101:635–642
Nascimento DS, Reis CU, Goldenberg RC, Ortiga-Carvalho TM, Pazos-Moura CC, Guggino SE, Guggino WB, Morales MM (2003) Estrogen modulates ClC-2 chloride channel gene expression in rat kidney. Pflugers Arch 446:593–599
Santos Ornellas D, Grozovsky R, Goldenberg RC, Carvalho DP, Fong P, Guggino WB, Morales M (2003) Thyroid hormone modulates ClC-2 chloride channel gene expression in rat renal proximal tubules. J Endocrinol 178:503–511
Arreola J, Begenisch T, Nehrke K, Nguyen HV, Park K, Richardson L, Yang B, Schutte BC, Lamb FS, Melvin JE (2002) Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl− channel gene. J Physiol 545(1):207–216
Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE (2002) Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem 26:23604–23611
Steinmeyer K, Ortland C, Jentsch TJ (1991) Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature 354:301–304
Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ (2000) ClC-5 Cl− channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 408:369–373
Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB (2000) Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9:2937–2945
Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ (1995) Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J Biol Chem 270:31172–31177
Vandewalle A, Cluzeaud F, Peng KC, Bens M, Lüchow A, Günther W, Jentsch TJ (2001) Tissue distribution and subcellular localization of the ClC-5 chloride channel in rat intestinal cells. Am J Physiol Cell Physiol 280:C373–C381
Sakamoto H, Sado Y, Naito I, Kwon TH, Inoue S, Endo K, Kawasaki M, Uchida S, Nielsen S, Sasaki S, Marumo F (1999) Cellular and subcellular immunolocalization of ClC-5 channel in mouse kidney: colocalization with H+-ATPase. Am J Physiol 277:F957–F965
Devuyst O, Christie PT, Courtoy PJ, Beauwens R, Thakker RV (1999) Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent’s disease. Hum Mol Genet 8:247–257
Schwake M, Friedrich T, Jentsch TJ (2001) An internalization signal in ClC-5, an endosomal Cl− channel mutated in Dent’s disease. J Biol Chem 276:12049–12054
Friedrich T, Breiderhoff T, Jentsch TJ (1999) Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J Biol Chem 274:896–902
Zdebik AA, Zifarelli G, Bergsdorf E-Y, Soliani P, Scheel O, Jentsch TJ, Pusch M (2008) Determinants of anion–proton coupling in mammalian endosomal CLC proteins. J Biol Chem 283:4219–4227
Accardi A, Walden M, Nguitragool W, Jayaram H, Williams C, Miller C (2005) Separate ion pathways in a Cl−/H+ exchanger. J Gen Physiol 126:563–570
Hebeisen S, Heidtmann H, Cosmelli D, González C, Poser B, Latorre R, Alvarez O, Fahlke C (2003) Anion permeation in human ClC-4 channels. Biophys J 84:2306–2318
Wrong OM, Norden AG, Feest TG (1994) Dent’s disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Q J Med 87:473–493
Lloyd SE, Günther W, Pearce SH, Thomson A, Bianchi ML, Bosio M, Craig IW, Fisher SE, Scheinman SJ, Wrong O, Jentsch TJ, Thakker RV (1997) Characterisation of renal chloride channel, CLCN5, mutations in hypercalciuric nephrolithiasis (kidney stones) disorders. Hum Mol Genet 6:1233–1239
Morimoto T, Uchida S, Sakamoto H, Kondo Y, Hanamizu H, Fukui M, Tomino Y, Nagano N, Sasaki S, Marumo F (1998) Mutations in CLCN5 chloride channel in Japanese patients with low molecular weight proteinuria. J Am Soc Nephrol 9:811–818
Ludwig M, Doroszewicz J, Seyberth HW, Bokenkamp A, Balluch B, Nuutinen M, Utsch B, Waldegger S (2005) Functional evaluation of Dent’s disease-causing mutations: implications for ClC-5 channel trafficking and internalization. Hum Genet 117:228–237
Wu F, Roche P, Christie PT, Loh NY, Reed AA, Esnouf RM, Thakker RV (2003) Modeling study of human renal chloride channel (hCLC-5) mutations suggests a structural–functional relationship. Kidney Int 63:1426–1432
Wang Y, Cai H, Cebotaru L, Hryciw DH, Weinman EJ, Donowitz M, Guggino SE, Guggino WB (2005) ClC-5: role in endocytosis in the proximal tubule. Am J Physiol Renal Physiol 289:F850–F862
Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ (2003) Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A 100:8472–8477
Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE (1999) Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155:1361–1370
Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515
Christensen EI, Birn H (2002) Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3:256–266
Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI (2007) Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proc Natl Acad Sci U S A 104:5407–5412
Mellman I (1996) Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12:575–625
Clague MJ, Urbe S, Aniento F, Gruenberg J (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269:21–24
Maranda B, Brown D, Bourgoin S, Casanova JE, Vinay P, Ausiello DA, Marshansky V (2001) Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J Biol Chem 276:18540–18550
Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Gekle M, Mildenberger S, Freudinger R, Silbernagl S (1995) Endosomal alkalinization reduces Jmax and Km of albumin receptor- mediated endocytosis in OK cells. Am J Physiol 268:F899–F906
Presley JF, Mayor S, McGraw TE, Dunn KW, Maxfield FR (1997) Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J Biol Chem 272:13929–13936
Forster IC, Hernando N, Biber J, Murer H (2006) Proximal tubular handling of phosphate: a molecular perspective. Kidney Int 70:1548–1559
Murer H, Forster I, Hernando N, Lambert G, Traebert M, Biber J (1999) Posttranscriptional regulation of the proximal tubule NaPi-II transporter in response to PTH and dietary Pi. Am J Physiol 277:F676–F684
Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW, Thakker RV, Unwin RJ, Wrong O (2001) Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int 60:1885–1892
Hilpert J, Nykjaer A, Jacobsen C, Wallukat G, Nielsen R, Moestrup SK, Haller H, Luft FC, Christensen EI, Willnow TE (1999) Megalin antagonizes activation of the parathyroid hormone receptor. J Biol Chem 274:5620–5625
Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, DeLuca HF (1998) Parathyroid hormone activation of the 25-hydroxyvitamin D3-1α-hydroxylase gene promoter. Proc Natl Acad Sci U S A 95:1387–1391
Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T, Kato S (1999) Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2D3 in intact animals. Endocrinology 140:2224–2231
Zierold C, Mings JA, DeLuca HF (2001) Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc Natl Acad Sci U S A 98:13572–13576
Wright J, Morales MM, Sousa-Menzes J, Ornellas D, Sipes J, Cui Y, Cui I, Hulamm P, Cebotaru V, Cebotaru L, Guggino WB, Guggino SE (2008) Transcriptional adaptation to Clcn5 knockout in proximal tubules of mouse kidney. Physiol Genomics 33:341–354
Scheinman SJ (1998) X-linked hypercalciuric nephrolithiasis: clinical syndromes and chloride channel mutations. Kidney Int 53:3–17
Silva IV, Cebotaru V, Wang H, Wang XT, Wang SS, Guo G, Devuyst O, Thakker RV, Guggino WB, Guggino SE (2003) The ClC-5 knockout mouse model of Dent’s disease has renal hypercalciuria and increased bone turnover. J Bone Miner Res 18:615–623
Hoopes RR Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ (2005) Dent disease with mutations in OCRL1. Am J Hum Genet 76:260–267
Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P (2007) A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell 13:377–390
Hoopes RR Jr, Raja KM, Koich A, Hueber P, Reid R, Knohl SJ, Scheinman SJ (2004) Evidence for genetic heterogeneity in Dent’s disease. Kidney Int 65:1615–1620
Moulin P, Igarashi T, Van Der Smissen P, Cosyns JP, Verroust P, Thakker RV, Scheinman SJ, Courtoy PJ, Devuyst O (2003) Altered polarity and expression of H+-ATPase without ultrastructural changes in kidneys of Dent’s disease patients. Kidney Int 63:1285–1295
Souza-Menezes J, Morales M, Tukaye D, Guggino S, Guggino W (2007) Absence of ClC5 in knockout mice leads to glycosuria, impaired renal glucose handling and low proximal tubule GLUT2 protein expression. Cell Physiol Biochem 20:455–464
Gailly P, Jouret F, Martin D, Debaix H, Parreira KS, Nishita T, Blanchard A, Antignac C, Willnow TE, Courtoy PJ, Scheinman SJ, Christensen EI, Devuyst O (2008) A novel renal carbonic anhydrase type III plays a role in proximal tubule dysfunction. Kidney Int 74:52–61
Alioth S, Meyer S, Dutzler R, Pervushin K (2007) The cytoplasmic domain of the chloride channel ClC-0: structural and dynamic characterization of flexible regions. J Mol Biol 369:1163–1169
Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D (1996) WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 15:2371–2380
Pirozzi G, McConnell SJ, Uveges AJ, Carter JM, Sparks AB, Kay BK, Fowlkes DM (1997) Identification of novel human WW domain-containing proteins by cloning of ligand targets. J Biol Chem 272:14611–14616
Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, Cook DI, Pollock CA, Poronnik P (2004) Nedd4-2 functionally interacts with ClC-5: involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem 279:54996–55007
Hryciw DH, Wang Y, Devuyst O, Pollock CA, Poronnik P, Guggino WB (2003) Cofilin interacts with ClC-5 and regulates albumin uptake in proximal tubule cell lines. J Biol Chem 278:40169–40176
Hryciw DH, Ekberg J, Ferguson C, Lee A, Wang D, Parton RG, Pollock CA, Yun CC, Poronnik P (2006) Regulation of albumin endocytosis by PSD95/Dlg/ZO-1 (PDZ) scaffolds. Interaction of Na+–H+ exchange regulatory factor-2 with ClC-5. J Biol Chem 281:16068–16077
Cunningham R, Esmaili A, Brown E, Biswas RS, Murtazina R, Donowitz M, Dijkman HB, van der Vlag J, Hogema BM, De Jonge HR, Shenolikar S, Wade JB, Weinman EJ (2008) Urine electrolyte, mineral, and protein excretion in NHERF-2 and NHERF-1 null mice. Am J Physiol Renal Physiol 294:F1001–F1007
Jouret F, Bernard A, Hermans C, Dom G, Terryn S, Leal T, Lebecque P, Cassiman JJ, Scholte BJ, de Jonge HR, Courtoy PJ, Devuyst O (2007) Cystic fibrosis is associated with a defect in apical receptor-mediated endocytosis in mouse and human kidney. J Am Soc Nephrol 18:707–718
Ludwig M, Utsch B (2004) Dent disease-like phenotype and the chloride channel ClC-4 (CLCN4) gene. Am J Med Genet A 128A:434–435
Acknowledgments
We thank EI Christensen for allowing us to reproduce his EM picture in Fig. 3b. Work in our laboratory was supported by the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung und Forschung (BMBF) in the framework of the National Genome Project (NGFN2), the European Union, the Prix Louis-Jeantet de Médecine, and the Ernst-Jung-Preis für Medizin to TJJ. VP is supported by Marie Curie Fellowship of the European Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plans, V., Rickheit, G. & Jentsch, T.J. Physiological roles of CLC Cl−/H+ exchangers in renal proximal tubules. Pflugers Arch - Eur J Physiol 458, 23–37 (2009). https://doi.org/10.1007/s00424-008-0597-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-008-0597-z