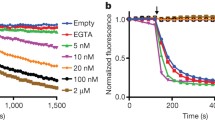
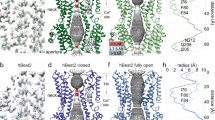
Abstract
Although known for more than 20 years, the molecular identity of epithelial Ca2+-activated Cl− channels remains obscure. Previous candidate proteins did not hold initial promises, and thus, new hope is put into the recently identified family of bestrophin proteins, as they reflect many of the properties found for native channels. Mutations in the bestrophin gene cause an autosomal form of macular dystrophy of the retina. Bestrophin 1 is assumed to form the basolateral Ca2+-activated Cl− channel in the retinal pigment epithelium of the eye. Other data suggest that bestrophin is a regulator of voltage gated Ca2+ channels. Structural information on bestrophins is available and a Cl− selective filter has been localized to the second transmembrane domain of bestrophin. It is possible that bestrophins function as physiologically regulated Cl− channels only in association with additional subunits and auxiliary proteins. Little is known about expression of bestrophin in gland acinar cells, which show a pronounced Ca2+-activated Cl− secretion. In airways and intestinal epithelia, bestrophins could be particularly important in diseases such as cystic fibrosis and secretory diarrhea.








Similar content being viewed by others
References
Ashton N, Evans RL, ElliottAC, Green R, Argent BE (1993) Regulation of fluid secretion and intracellular messengers in isolated rat pancreatic ducts by acetylcholine. J Physiol 471:549–562
Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101–104
Barro Soria R, Spitzner M, Schreiber R, Kunzelmann K (2007) Bestrophin 1 enables Ca2+ activated Cl− conductance in epithelia. J Biol Chem (in press)
Chien LT, Zhang ZR, Hartzell HC (2006) Single Cl− channels activated by Ca2+ in Drosophila S2 cells are mediated by bestrophins. J Gen Physiol 128:247–259
Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP (1997) The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A 94:3960–3965
Duan D, Winter C, Cowley S, Hume JR, Horowitz B (1997) Molecular identification of a volume-regulated chloride channel. Nature 390:417–421
Duta V, Szkotak AJ, Nahirney D, Duszyk M (2004) The role of bestrophin in airway epithelial ion transport. FEBS Lett 577:551–554
Eggermont J (2004) Calcium-activated chloride channels: (un)known, (un)loved? Proc Am Thorac Soc 1:22–27
Fischmeister R, Hartzell HC (2005) Volume sensitivity of the bestrophin family of chloride channels. J Physiol 562:477–491
Frings S, Reuter D, Kleene SJ (2000) Neuronal Ca2+-activated Cl− channels-homing in on an elusive channel species. Prog Neurobiol 60:247–289
Fujii S, Gallemore RP, Hughes BA, Steinberg RH (1992) Direct evidence for a basolateral membrane Cl− conductance in toad retinal pigment epithelium. Am J Physiol 262:C374–C383
Gabriel SE, McDaniel J, Wasilchen T, Kreda SM, Quinney N (2006) Bestrophin-mediated Ca2+ activated Cl− conductance of the airway epithelium. FASEB J 16:A256
Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV (2006) NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci 119:226–238
Greenwood IA (2004) CLC-3 knockout hints at swelling-activated chloride channel complexity. J Physiol 557:343
Greenwood IA, Leblanc N (2007) Overlapping pharmacology of Ca(2+)-activated Cl(−) and K(+) channels. Trends Pharmacol Sci 28:1–5
Greenwood IA, Miller LJ, Ohya S, Horowitz B (2002) The large conductance potassium channel beta-subunit can interact with and modulate the functional properties of a calcium-activated chloride channel, CLCA1. J Biol Chem 277:22119–22122
Hartzell HC, Putzier I, Arreola J (2005) Calcium-activated chloride channels. Annu Rev Physiol 67:719–758
Hartzell HC, Qu Z, Putzier I, Artinian L, Chien LT, Cui Y (2005) Looking chloride channels straight in the eye: bestrophins, lipofuscinosis, and retinal degeneration. Physiology (Bethesda) 20:292–302
Ho MW, Kaetzel MA, Armstrong DL, Shears SB (2001) Regulation of a human chloride channel. A paradigm for integrating input from calcium, type ii calmodulin-dependent protein kinase, and inositol 3,4,5,6-tetrakisphosphate. J Biol Chem 276:18673–18680
Huang P, Gilmore E, Kultgen P, Barnes P, Milgram S, Stutts MJ (2004) Local regulation of cystic fibrosis transmembrane regulator and epithelial sodium channel in airway epithelium. Proc Am Thorac Soc 1:33–37
Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ (2001) Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem 276:20093–20100
HuangY, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, Reinhold WC, Papp A, Weinstein JN, Sadee W (2004) Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res 64:4294–4301
Hughes BA, Gallemore, Gallemore RP, Miller SM (1998) Transport mechanisms in the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ (eds) The retinal pigment epithelium-function and disease. Oxford University Press 1:103–134
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2001) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568
Joo NS, Irokawa T, Robbins RC, Wine JJ (2006) Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem 281:7392–7398
Kidd JF, Thorn P (2000) Intracellular Ca2+ and Cl− channel activation in secretory cells. Annu Rev Physiol 62:493–513
Kreda SM, Mall M, Mengos A, Rochelle LG, Yankaskas J, Riordan JR, Boucher RC (2005) CFTR in human airways. Molec Biol Cell 16:2154–2167
Kunzelmann K (2005) Ion channels and cancer. J Membr Biol 205:159–173
Kunzelmann K, König J, Markovich D, King N, Karupiah G, Cook DI (2004) Acute effects of parainfluenza virus on epithelial electrolyte transport. J Biol Chem 279:48760–48766
Kunzelmann K, Mall M (2002) Electrolyte transport in the colon: Mechanisms and implications for disease. Physiol Rev 82:245–289
Kunzelmann K, Mall M (2003) Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics. Am J Respir Medicine 2:299–309
Kunzelmann K, Scheidt K, Scharf B, Ousingsawat J, Schreiber R, Wainwright BJ, McMorran B (2006) Pseudomonas flagellin inhibits Na+ transport in airway epithelia. FASEB J 20:545–546
Kunzelmann K, Sun J, Markovich D, König J, Mürle B, Mall M, Schreiber R (2005) Control of ion transport in mammalian airways by protease activated receptors type 2 (PAR-2). FASEB J 19:969–970
Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA (2005) Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharm 83:541–556
Leipziger J (2003) Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284:F419–F432
Loewen ME, Forsyth GW (2005) Structure and function of CLCA proteins. Physiol Rev 85:1061–1092
Mall M, Bleich M, Greger R, Schürlein M, Kühr J, Seydewitz HH, Brandis M, Kunzelmann K (1998) Cholinergic ion secretion in human colon requires co-activation by cAMP. Am J Physiol 275:G1274–G1281
Mall M, Wissner A, Kühr J, Gonska T, Brandis M, Kunzelmann K (2000) Inhibition of amiloride sensitive epithelial Na+ absorption by extracellular nucleotides in human normal and CF airways. Am J Respir Cell Mol Biol 23:755–761
Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K (2000) Bestrophin, the product of the best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A 97:12758–12763
Marmorstein LY, McLaughlin PJ, Stanton JB, Yan L, Crabb JW, Marmorstein AD (2002) Bestrophin interacts physically and functionally with protein phosphatase 2A. J Biol Chem 277(34):30591–30597
Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD (2006) The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (Best-1). J Gen Physiol 127:577–589
Melvin JE, Yule D, Shuttleworth T, Begenisich T (2005) Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67:445–469
Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela JM (2006) Generation of specific Ca(2+) signals from Ca(2+) stores and endocytosis by differential coupling to messengers. Curr Biol 16:1931–1937
Mergler S, Strauss O (2002) Stimulation of L-type Ca(2+) channels by increase of intracellular InsP3 in rat retinal pigment epithelial cells. Exp Eye Res 74:29–40
Milenkovic VM, Rivera A, Horling F, Weber BH (2007) Insertion and topology of normal and mutant bestrophin-1 in the endoplasmic reticulum membrane. J Biol Chem 282:1313–1321
Mizukawa Y, Nishizawa T, Nagao T, Kitamura K, Urushidani T (2002) Cellular distribution of parchorin, a chloride intracellular channel-related protein, in various tissues. Am J Physiol Cell Physiol 282:C786–C795
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Petersen OH (2005) Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium 38:171–200
Peterson WM, Meggyesy C, Yu K, Miller SS (1997) Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci 17:2324–2337
Pifferi S, Pascarella G, Boccaccio A, Mazzatenta A, Gustincich S, Menini A, and Zucchelli S (2006) Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci U S A 103:12929–12934
Puntheeranurak S, Schreiber R, Kunzelmann K, Krishnamra N (2007) Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem 19:77–88
Qu Z, Chien LT, Cui Y, Hartzell HC (2006) The anion-selective pore of the bestrophins, a family of chloride channels associated with retinal degeneration. J Neurosci 26:5411–5419
Qu Z, Fischmeister R, Hartzell HC (2004) Mouse bestrophin-2 is a bona fide Cl(−) channel: identification of a residue important in anion binding and conduction. J Gen Physiol 123:327–340
Qu Z, Hartzell HC (2004) Determinants of anion permeation in the second transmembrane domain of the mouse bestrophin-2 chloride channel. J Gen Physiol 124:371–382
Qu Z, Wei RW, Mann W, Hartzell HC (2003) Two bestrophins cloned from Xenopus laevis Oocytes express Ca-activated Cl currents. J Biol Chem 278:49563–49572
Reigada D, Lu W, Mitchell CH (2006) Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J Physiol 575:707–720
Ritzka M, Stanke F, Jansen S, Gruber AD, Pusch L, Woelfl S, Veeze HJ, Halley DJ, Tummler B (2004) The CLCA gene locus as a modulator of the gastrointestinal basic defect in cystic fibrosis. Hum Genet 115:483–491
Rosenthal R, Bakall B, Kinnick T, Peachey N, Wimmers S, Wadelius C, Marmorstein AD, Strauss O (2005) Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J 20:178–180
Schlatter E, Greger R, Schafer JA (1990) Principal cells of cortical collecting ducts of the rat are not a route of transepithelial Cl− transport. Pflugers Arch 417:317–323
Schreiber R, Kunzelmann K (2005) Purinergic P2Y6 receptors induce Ca2+ and CFTR dependent Cl− secretion in mouse trachea. Cell Physiol Biochem 16:99–108
Stanton JB, Goldberg AF, Hoppe G, Marmorstein LY, Marmorstein AD (2006) Hydrodynamic properties of porcine bestrophin-1 in Triton X-100. Biochim Biophys Acta 1758:241–247
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881
Sun H, Tsunenari T, Yau KW, Nathans J (2001) The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A 99:4008–4013
Suzuki M (2006) The Drosophila tweety family: molecular candidates for large-conductance Ca2+-activated Cl− channels. Exp Physiol 91:141–147
Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredburg JJ, Boucher RC (2005) Normal and cystic fbrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 280:35751–35759
Tarran R, Loewen ME, Paradiso AM, Olsen JC, Gray MA, Argent BE, Boucher RC, Gabriel SE (2002) Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl− conductances. J Gen Physiol 120:407–418
Tsunenari T, Nathans J, Yau KW (2006) Ca2+-activated Cl− current from human bestrophin-4 in excised membrane patches. J Gen Physiol 127:749–754
Tsunenari T, Sun H, Williams J, Cahill H, Smallwood P, Yau KW, Nathans J (2003) Structure-function analysis of the bestrophin family of anion channels. J Biol Chem 278:41114–41125
Winpenny JP, Harris A, Hollingsworth MA, Argent BE, Gray MA (1998) Calcium-activated chloride conductance in a pancreatic adenocarcinoma cell line of ductal origin (HPAF) and in freshly isolated human pancreatic duct cells. Pflugers Arch 435:796–803
Yamamoto-Mizuma S, Wang GX, Liu LL, Schegg K, Hatton WJ, Duan D, Horowitz TL, Lamb FS, Hume JR (2004) Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3−/− mice. J Physiol 557:439–456
Yu K, Cui Y, Hartzell HC (2006) The bestrophin mutation A243V, linked to adult-onset vitelliform macular dystrophy, impairs its chloride channel function. Invest Ophthalmol Vis Sci 47:4956–4961
Acknowledgement
This work is continuously supported by the Deutsche Forschungsgemeinschaft DFG SFB699 A7 and the Else-Kröner-Fresenius-Stiftung. We acknowledge the expert technical assistances by Ms. E. Tartler and Ms. A. Paech. Bestrophins were a generous gift from Prof. Dr. J. Nathans (Howard Hughes Medical Institute, Johns Hopkins University School of Medicine, Baltimore, USA). The C2-toxin and HEK293 cells were kindly provided by Prof. Dr. R. Benz (Biozentrum der Universität Würzburg) and Prof. Dr. R. Witzgall (University of Regensburg, Germany). The GFP-tagged hBest-1 was kindly provided by Prof. Dr. B.H.F. Weber (Institute of Human Genetics, University of Regensburg, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunzelmann, K., Milenkovic, V.M., Spitzner, M. et al. Calcium-dependent chloride conductance in epithelia: is there a contribution by Bestrophin?. Pflugers Arch - Eur J Physiol 454, 879–889 (2007). https://doi.org/10.1007/s00424-007-0245-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0245-z