Abstract
Purpose
Indocyanine green fluorescence angiography (ICG-FA) is an established technique for assessment of intestinal perfusion during gastrointestinal surgery, whereas quantitative ICG-FA (q-ICG) and laser speckle contrast imaging (LSCI) are relatively unproven. The study aimed to investigate whether the techniques could be applied interchangeably for perfusion assessment.
Methods
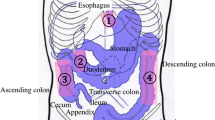
Nineteen pigs underwent laparotomy, two minor resections of the small bowel, and anastomoses. Additionally, seven pigs had parts of their stomach and small intestine de-vascularized. Data was also collected from an in vivo model (inferior caval vein measurements in two additional pigs) and an ex vivo flow model, allowing for standardization of experimental flow, distance, and angulation. Q-ICG and LSCI were performed, so that regions of interest were matched between the two modalities in the analyses, ensuring coverage of the same tissue.
Results
The overall correlation of q-ICG and LSCI evaluated in the porcine model was modest (rho = 0.45, p < 0.001), but high in tissue with low perfusion (rho = 0.74, p < 0.001).
Flux values obtained by LSCI from the ex vivo flow model revealed a decreasing flux with linearly increasing distance as well as angulation to the model. The Q-ICG perfusion values obtained varied slightly with increasing distance as well as angulation to the model.
Conclusions
Q-ICG and LSCI cannot be used interchangeably but may supplement each other. LSCI is profoundly affected by angulation and distance. In comparison, q-ICG is minimally affected by changing experimental conditions and is more readily applicable in minimally invasive surgery.






Similar content being viewed by others
Abbreviations
- ICG:
-
Indocyanine green
- ICG-FA:
-
Indocyanine green–fluorescence angiography
- LSCI:
-
Laser speckle contrast imaging
- LSPU:
-
Laser speckle perfusion units
- q-ICG:
-
Quantitative indocyanine green fluorescence angiography
- ROI:
-
Region of interest
- RPM:
-
Revolutions per minute
References
Thompson SK, Chang EY, Jobe BA (2006) Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery 26:131–136. https://doi.org/10.1002/micr.20197
Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, Margolin DA, Martz JE, McLemore EC, Molena D, Newman MI, Rafferty JF, Safar B, Senagore AJ, Zmora O, Wexner SD (2016) Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg 20:2035–2051. https://doi.org/10.1007/s11605-016-3255-3
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Kofoed SC, Calatayud D, Jensen LS, Helgstrand F, Achiam MP, De Heer P, Svendsen LB (2015) Intrathoracic anastomotic leakage after gastroesophageal cancer resection is associated with increased risk of recurrence. J Thorac Cardiovasc Surg 150:42–48. https://doi.org/10.1016/j.jtcvs.2015.04.030
Kofoed SC, Calatayud D, Jensen LS, Jensen MV, Svendsen LB (2014) Intrathoracic anastomotic leakage after gastroesophageal cancer resection is associated with reduced long-term survival. World J Surg 38:114–119. https://doi.org/10.1007/s00268-013-2245-9
Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM (2010) Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal disease : the official journal of the Association of. Coloproctol G B Irel 12:1018–1025. https://doi.org/10.1111/j.1463-1318.2009.01944.x
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Color Dis 24:569–576. https://doi.org/10.1007/s00384-009-0658-6
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–899. https://doi.org/10.1097/SLA.0b013e3182128929
Jessen M, Nerstrom M, Wilbek TE, Roepstorff S, Rasmussen MS, Krarup PM (2016) Risk factors for clinical anastomotic leakage after right hemicolectomy. Int J Color Dis 31:1619–1624. https://doi.org/10.1007/s00384-016-2623-5
Briers JD, Fercher AF (1982) Retinal blood-flow visualization by means of laser speckle photography. Invest Ophthalmol Vis Sci 22:255–259
Owens SL (1996) Indocyanine green angiography. Br J Ophthalmol 80:263–266
Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA (2012) Digital indocyanine green videoangiography and choroidal neovascularization. 1992. Retina 32(Suppl 1):191
Valdes PA, Roberts DW, Lu FK, Golby A (2016) Optical technologies for intraoperative neurosurgical guidance. Neurosurg Focus 40:E8. https://doi.org/10.3171/2015.12.focus15550
Fredrickson VL, Russin JJ, Strickland BA, Bakhsheshian J, Amar AP (2017) Intraoperative imaging for vascular lesions. Neurosurg Clin N Am 28:603–613. https://doi.org/10.1016/j.nec.2017.05.011
Kaiser M, Yafi A, Cinat M, Choi B, Durkin AJ (2011) Noninvasive assessment of burn wound severity using optical technology: a review of current and future modalities. Burns 37:377–386. https://doi.org/10.1016/j.burns.2010.11.012
Alander JT, Kaartinen I, Laakso A, Patila T, Spillmann T, Tuchin VV, Venermo M, Valisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012:940585. https://doi.org/10.1155/2012/940585
Marano A, Priora F, Lenti LM, Ravazzoni F, Quarati R, Spinoglio G (2013) Application of fluorescence in robotic general surgery: review of the literature and state of the art. World J Surg 37:2800–2811. https://doi.org/10.1007/s00268-013-2066-x
Degett TH, Andersen HS, Gogenur I (2016) Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbeck's Arch Surg 401:767–775. https://doi.org/10.1007/s00423-016-1400-9
Nerup N, Andersen HS, Ambrus R, Strandby RB, Svendsen MBS, Madsen MH, Svendsen LB, Achiam MP (2017) Quantification of fluorescence angiography in a porcine model. Langenbeck's Arch Surg 402:655–662. https://doi.org/10.1007/s00423-016-1531-z
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102:e169–e176. https://doi.org/10.1002/bjs.9725
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31:4184–4193. https://doi.org/10.1007/s00464-017-5475-3
Toens C, Krones CJ, Blum U, Fernandez V, Grommes J, Hoelzl F, Stumpf M, Klinge U, Schumpelick V (2006) Validation of IC-VIEW fluorescence videography in a rabbit model of mesenteric ischaemia and reperfusion. Int J Color Dis 21:332–338. https://doi.org/10.1007/s00384-005-0017-1
Nadort A, Kalkman K, van Leeuwen TG, Faber DJ (2016) Quantitative blood flow velocity imaging using laser speckle flowmetry. Sci Rep 6:25258. https://doi.org/10.1038/srep25258
Forrester KR, Tulip J, Leonard C, Stewart C, Bray RC (2004) A laser speckle imaging technique for measuring tissue perfusion. IEEE Trans Biomed Eng 51:2074–2084. https://doi.org/10.1109/TBME.2004.834259
Senarathna J, Rege A, Li N, Thakor NV (2013) Laser speckle contrast imaging: theory, instrumentation and applications. IEEE Rev Biomed Eng 6:99–110. https://doi.org/10.1109/RBME.2013.2243140
Ambrus R, Strandby RB, Svendsen LB, Achiam MP, Steffensen JF, Sondergaard Svendsen MB (2016) Laser speckle contrast imaging for monitoring changes in microvascular blood flow. Eur Surg Res 56:87–96. https://doi.org/10.1159/000442790
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. https://doi.org/10.1371/journal.pbio.1000412
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259:700–707. https://doi.org/10.1097/SLA.0b013e31828d4ab3
Mukaka MM (2012) Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24:69–71
Lee IA, Preacher KJ (2013) Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software]. Available from http://quantpsy.org. Accessed 11 Jan 2018
Steiger JH (1980) Tests for comparing elements of a correlation matrix. Psychol Bull 87:245–251. https://doi.org/10.1037/0033-2909.87.2.245
Davis MA, Kazmi SM, Dunn AK (2014) Imaging depth and multiple scattering in laser speckle contrast imaging. J Biomed Opt 19:086001. https://doi.org/10.1117/1.jbo.19.8.086001
Frangioni JV (2008) New technologies for human cancer imaging. J Clin Oncol 26:4012–4021. https://doi.org/10.1200/jco.2007.14.3065
Ambrus R, Strandby RB, Secher NH, Runitz K, Svendsen MB, Petersen LG, Achiam MP, Svendsen LB (2016) Thoracic epidural analgesia reduces gastric microcirculation in the pig. BMC Anesthesiol 16:86. https://doi.org/10.1186/s12871-016-0256-4
Heymann MA, Payne BD, Hoffman JI, Rudolph AM (1977) Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20:55–79
Lange M, Hamahata A, Traber DL, Nakano Y, Traber LD, Enkhbaatar P (2013) Multiple versus single injections of fluorescent microspheres for the determination of regional organ blood flow in septic sheep. Lab Anim 47:203–209. https://doi.org/10.1177/0023677213487718
Reinhardt CP, Dalhberg S, Tries MA, Marcel R, Leppo JA (2001) Stable labeled microspheres to measure perfusion: validation of a neutron activation assay technique. Am J Physiol Heart Circ Physiol 280:H108–H116
Alemanno G, Somigli R, Prosperi P, Bergamini C, Maltinti G, Giordano A, Valeri A (2016) Combination of diagnostic laparoscopy and intraoperative indocyanine green fluorescence angiography for the early detection of intestinal ischemia not detectable at CT scan. Int J Surg Case Rep 26:77–80. https://doi.org/10.1016/j.ijscr.2016.07.016
Shimizu S, Kamiike W, Hatanaka N, Yoshida Y, Tagawa K, Miyata M, Matsuda H (1995) New method for measuring ICG Rmax with a clearance meter. World J Surg 19:113–118 discussion 118
Nerup N, Knudsen KBK, Ambrus R, Svendsen MBS, Thymann T, Ifaoui IBR, Svendsen LB, Achiam MP (2017) Reproducibility and reliability of repeated quantitative fluorescence angiography. Surg Technol Int 31:35–39
Quero G, Lapergola A, Barberio M, Seeliger B, Saccomandi P, Guerriero L, Mutter D, Saadi A, Worreth M, Marescaux J, Agnus V, Diana M (2018) Discrimination between arterial and venous bowel ischemia by computer-assisted analysis of the fluorescent signal. Surg Endosc. https://doi.org/10.1007/s00464-018-6512-6
Baiocchi GL, Diana M, Boni L (2018) Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: state of the art and future directions. World J Gastroenterol 24:2921–2930. https://doi.org/10.3748/wjg.v24.i27.2921
Ortega AE, Richman MF, Hernandez M, Peters JH, Anthone GJ, Azen S, Beart RW Jr (1996) Inferior vena caval blood flow and cardiac hemodynamics during carbon dioxide pneumoperitoneum. Surg Endosc 10:920–924
Lindberg F, Bergqvist D, Rasmussen I, Haglund U (1997) Hemodynamic changes in the inferior caval vein during pneumoperitoneum. An experimental study in pigs. Surg Endosc 11:431–437
Acknowledgments
The study was sponsored by donations from private foundations, to whom the authors owe gratitude: Mogens Andresen fonden, Civilingeniør Johannes Elmqvist Ormstrup og Hustru Grete Omstrups Fond, and Fabrikant Frands Køhler Nielsens og Hustrus Mindelegat. Sponsors had no role in study design, interpretation of results, or any other part of the study. Also, a sincere thanks to Jens Osterkamp, MD, for the help with illustrations.
Author information
Authors and Affiliations
Contributions
Study conception and design: JHR, NN, LBS, MPA. Acquisition of data: JHR, RA, NN, RBS. Analysis and interpretation of data: JHR, NN, RBS, MBS, RA, LBS, MPA. Drafting of the manuscript: JHR, NN. Critical revision and final approval of the manuscript: JHR, NN, RBS, MBS, RA, LBS, MPA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rønn, J.H., Nerup, N., Strandby, R.B. et al. Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbecks Arch Surg 404, 505–515 (2019). https://doi.org/10.1007/s00423-019-01789-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-019-01789-8