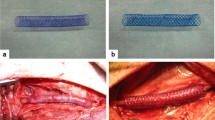
Abstract
Background and aims
External support of vein grafts by fibrin glue possibly prevents overdistension, vascular remodeling, and neointimal hyperplasia. Previous animal models of neointimal hyperplasia showed conflicting results. Here, long-term effects of external fibrin glue support were studied in a new rat model of jugular vein to abdominal aorta transposition.
Materials and methods and methods
In male Wistar rats (250–300 g) right jugular vein (1.0–1.5 cm) was transposed to the infrarenal aorta. Fibrin glue (0.25 ml) covered the vein before releasing the vascular clamps (n = 6). Control vein grafts were exposed directly to blood pressure. After 16 weeks vein grafts were pressure-fixed for histology. Intima thickness, luminal and intimal area were measured by planimetry and elastic fibers demonstrated by Elastica van Giesson staining.
Results
Intimal thickness (74.04 ± 6.7 µm vs 1245 ± 187 µm, control vs fibrin treatment; p < 0.001), intimal area (2517.16 ± 355 mm2 vs 18424 ± 4927 mm2, control vs fibrin treatment; p < 0.05) and luminal area (2184.75 ± 347 mm2 vs 7231.85 ± 1782 mm2, control vs fibrin treatment; p < 0.05) were significantly increased, elastic fibers in the vessel wall were diminished and the vessel wall infiltrated by mononuclear cells in fibrin glue supported veins.
Conclusion
External support of vein grafts by fibrin glue leads to aneurysmal degeneration and intimal hyperplasia, thereby possibly jeopardizing long-term graft patency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vein grafts for bypass grafting in vascular and cardiovascular surgery exert good long-term patency and show far better patency rates than synthetic grafts in peripheral vascular surgery [1]. However, after 10 years 40% of vein grafts in coronary artery surgery [2] and 50% of peripheral venous grafts fail, mainly due to the development of neointimal hyperplasia, especially at sites of anastomosis [3]. Vein graft damage after implantation is believed to be due to arterial pressure, thereby increasing wall tension, shear stress and pulsatile blood flow. This ultimately leads to overdistension of the vein graft promoting intimal hyperplasia through a remodeling process, which involves proliferation and migration of vascular smooth muscle cells (VSMC) as well as myofibroblast to the vein graft [4]. To reduce wall shear stress and to prevent the proposed negative effects of early vein graft distension due to arterial pressure, several ‘extra vascular support’ strategies have been introduced. These include the placement of a porous, non-restrictive, polyester stent [5–7], or a bio absorbable sheath [8] or the use of perivenous application of fibrin glue [9]. Perivenous fibrin glue has been used in a variety of experimental models and for different follow-up times [10–12]. The results of these studies were conflicting in terms of the development of intimal hyperplasia at different time points. Because endpoints were relatively early after venous grafting we sought to test longer follow-up times in a new model of arterialized jugular vein transposition to the infrarenal aorta.
Material and methods
Animals
Male Wistar rats, body weight 250–350 g, were purchased from Charles River Laboratories (Sulzfeld, Germany). Rats were fed standard laboratory chow and were kept according to the German legislation on the protection of animals at a 12-h day-and-night shift. The protocol was approved by the local authorities on the protection of animals (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit/Tierschutz, AZ: 33.42502–046/06, 29.06.06).
Operative procedure
This model has been used in mice, but has not been described in rats and offers a complete arterialized graft exposed to the arterial blood pressure [13].
After Sevoflurane anesthesia, right jugular vein was freed from surrounding tissue and small vein branches. It was then harvested at a length of 1.0–1.5 cm. The vein was flushed gently free from blood with 4°C Ringer solution with a syringe and stored in cold Ringer solution until implantation. Then the abdominal aorta was separated from the infrarenal cava vein. A corresponding segment of 1-cm aorta was excised and the jugular vein interposed with interrupted 8-0 nylon sutures.
Experimental design
N = 6 animals each were assigned to the following groups:
Fibrin group
Before declamping 0.25 ml of Fibrin glue (Tissucol Duo S 0.5 ml Immuno, Baxter Deutschland GmbH Edisonstraße 4 85716 Unterschleißheim, Germany) was applied onto the transposed vein graft. Then the blood flow was restored. Animals were followed for 16 weeks and killed thereafter.
Control group
Blood flow was restored without application of Fibrin glue. Animals were followed as described for the Fibrin group.
Fibrin control group: the jugular vein was freed as for transposition but left in place and covered with 0.25 ml of Fibrin glue (Tissucol Duo S 0.5 ml Immuno, Baxter Deutschland GmbH Edisonstraße 4 85716 Unterschleißheim, Germany)
Histology and morphometry
The vein segment was pressure fixated for 15 min with 4% Phosphate buffered Formalin at a hydrostatic pressure of 100 mmHg by flushing the formalin through the abdominal aorta replacing the entire blood volume by formalin. The probes were then stored for 36 h in formalin. For wax embedding, three segments were removed from each vein graft, dehydrated, and embedded in paraffin wax with their axis perpendicular to the cutting plane. Sections were stained with hematoxylin and eosin and elastic van Gieson for examination under light microscopy.
Each segment was analyzed in a blinded fashion. Vessel wall dimensions, intimal and luminal areas were measured by computer-aided planimetry with use of an Olympus® BH-2 microscope video camera, head (JVC TK-870E), computer (Victor V386A, Victor Technologies), SoftaOlympus DP-Soft 3.1® software and image-analysis system (Olympus Europe Holding GmbH, Hamburg, Germany). The area enclosed by the endothelium defined the lumen. The area enclosed between the internal elastic lamina and the lumen defined the intima. Measurements of perimeters of the lumen, intimal thickness, and total wall thickness were obtained. Mean values of luminal and intimal circumferences, as well as intimal and total wall thickness, were then calculated for all sections from the same graft.
Statistics
All values are presented as mean ± standard error of the mean (SEM). Data were analyzed for statistical difference by aid of the computer software GraphPad Instat® Version 3.05. Non-parametric statistic test Mann–Whitney was used to detect statistical differences. A two-tailed p value < 0.05 was accepted statistically significant.
Results
Morphology
Fibrin supported vessels showed a dense infiltration of mononuclear cells and foam cells and a disruption of vessel architecture. Neointimal formation was demonstrated in both groups, but fibrin glue supported veins showed an excessive formation of neointima and aneurysmal degeneration in four of six animals (Fig. 3A–C). Control vessels, in comparison, showed a preserved vessel architecture with no or minimal infiltration of mononuclear cells and much less development of intimal hyperplasia (Fig. 3D, E). Analyzing the fibrin control group without transposition revealed a damage in vessel wall architecture showing a scattered appearance with some degree of inflammation (Fig 3F)
Luminal area
Analyzing the total luminal area by computer-aided planimetry showed a significant increase in the fibrin-treated group with aneurysmal degeneration in four of six animals, 2,184.75 ± 347 mm2 vs 7,231.85 ± 1782 mm2, control vs fibrin treatment; p < 0.05 (Fig. 1, first column).
Planimetry of luminal area (µm2), enclosed by the endothelium and intimal area, which is defined as the area enclosed between the internal elastic lamina and the lumen. Control grafts (black columns) without external support vs fibrin glue (white columns) externally supported grafts. P < 0.05 significant difference between the respective groups
Intimal area
In addition to the increase of luminal area, the intima showed a significant increase in the intimal area measured in the fibrin groups compared to control, which also showed development of neointima, 2,517.16 ± 356 mm2 vs 18,424 ± 4,927 mm2, control vs fibrin treatment; p < 0.05, (Fig. 1, second column).
Total wall thickness
Parallel to the increase in luminal area, the total wall thickness in the fibrin-supported veins showed a significant increase in thickness compared to control, 273 ± 24 μm vs1,708 ± 211 μm control vs fibrin treatment; p < 0.05, (Fig. 2A).
A total wall thickness (µm) measured in unsupported control grafts (white columns) and fibrin glue externally supported grafts (black columns). p < 0.001 significant difference between the respective groups. B Intima thickness (µm) measured in unsupported control grafts (white columns) and fibrin glue externally supported grafts (black columns). p < 0.001 significant difference between the respective groups
Intimal thickness
Corresponding to the increase in total wall thickness and increase in intimal area, the intima thickened significantly in fibrin-supported veins compared to control grafts with a lesser degree of neointimal proliferation 74.04 ± 6.7 μm vs 1245 ± 187 μm control vs fibrin treatment; p < 0.05 (Fig. 2B).
Elastin fibers
Macroscopically, the elastin fibers in fibrin-supported veins were strongly diminished compared to control and foam cells were present only in fibrin-treated veins (Fig. 4). In the fibrin control group without transposition, elastic fibers were disrupted, causing loss of continuity within the vessel wall (Fig. 4C).
Discussion
Vein graft failure due to intimal hyperplasia jeopardizes long-term patency of vein grafts in peripheral vascular and cardiovascular surgery. Early graft damage is believed to be due to surgical trauma and overdistension-inducing mechanisms, which ultimately lead to intimal hyperplasia. Therefore, protecting vein grafts from early pressure-related distension injury is considered an important step toward longer vein graft function. External fibrin glue support could possibly counteract the intravenous arterial pressure thereby possibly preventing early vein graft overdistension. On the other hand, fibrin application could be used as a vehicle to transport therapeutic agents to prevent intimal hyperplasia at the site of action [14].
In a pig model of neointimal hyperplasia following balloon angioplasty-related endothelial injury, local application of losartan, an inhibitor of the renin–angiotensin system, could reduce neointimal hyperplasia [14]. However, the data deducted from this arterial injury model cannot necessarily be applied to our vein graft model because of the different pathophysiological cause, local endothelial damage due to balloon inflation vs generalized intraluminal pressure increase due to arterialization. Furthermore, the different histologies of the vessel wall between arteries and vein grafts (Figs. 3 and 4) may lead to different fibrin glue concentrations within the vessel wall compartments and may exert different effects. Previous in vitro experiments have revealed that early distension could be diminished by external fibrin glue support [7, 9, 11] and in vivo experiments have furthermore revealed that perivenous support of vein grafts with fibrin glue can attenuate the severe injury encountered in the non-supported vein grafts exposed to arterial pressure at early time points after grafting [10].
A Histological section stained by hematoxylin and eosin showing a cross-section through the vein graft externally supported by fibrin glue with intense neointimal formation (×25 magnification). B Histological section stained by hematoxylin and eosin of a cross-section through the vein graft externally supported by fibrin glue showing adventitial destruction of an aneurysmatically degenerated vein graft (×25 magnification) C Detailed view of a histological section stained by hematoxylin and eosin of the vessel wall of a fibrin glue externally supported vein graft showing dense infiltration of mononuclear cells and foam cells (arrows) (×100 magnification) D Histological section stained by hematoxylin and eosin of a cross-section through an unsupported control vein graft showing a normal appearance without adventitial destruction, aneurysmal degeneration but intimal hyperplasia (×6.5 magnifications) E Histological section stained by hematoxylin and eosin of a cross-section through an unsupported control vein graft with a more detailed view on intimal hyperplasia (×25 magnifications) F Histological section stained by hematoxylin and eosin of a cross-section through a fibrin control vein graft not transposed showing a disrupted vessel wall architecture (×25 magnifications)
A Histological section stained by Elastica van Giesson of a fibrin glue externally supported vein graft showing a widespread loss of elastic fibers within the vessel wall (×100 magnification) B Histological section stained by Elastica van Giesson of an unsupported control graft showing preserved elastic fibers within the vessel wall (×100 magnification) C Histological section stained by Elastica van Giesson of a fibrin control graft showing disrupted elastic fibers within the vessel wall and some degree of inflammation (×100 magnification)
However, at later time points it was demonstrated that external fibrin support showed negative or even detrimental effects on vein grafts [12]. In the experiments reported here, we could demonstrate a negative effect of external vein graft support in the rat. We used a new rat model of jugular vein transposition, which displayed a neointimal formation at 16 weeks follow-up. In our hands, external fibrin support leads to aneurysmal degeneration, excessive neointimal formation, and to an inflammatory response with the detection of foam cells and loss of elastin fibers. Control grafts also showed neointimal formation but without disruption of vessel wall architecture or aneurysmal degeneration.
In the fibrin control group without transposition, a similar disruption of elastic fibers within the vein graft was noted pointing toward a detrimental effect of fibrin on vessel wall architecture. This is in conjunction to previous reports in a pig model [12] showing an interspecies reproducible effect. It was stated that inflammation and infiltration of macrophages to vascular tissue is associated with fibrin [15]. As we could demonstrate the appearance of foam cells in fibrin supported grafts, we therefore hypothesize that in the process of fibrin degradation the inflammatory response with accumulation of macrophages and excretion of a variety of cytokines and elastases leads to lysis of elastin fibers. This could explain aneurysmal degeneration and intense accumulation of neointimal hyperplasia because in a distorted vessel architecture blood flow cannot be expected to be laminar, and vessel wall shear stress is likely to be greater and not predictable, leading to higher adaptive responses of the vessel and to excessive neointimal formation.
We conclude that in our hands external support of vein grafts by application of fibrin glue does not prevent neointimal hyperplasia in the long term, but, on the contrary, leads to an intense neointimal hyperplasia possibly due to the inflammatory response leading to cellular proliferation (smooth muscle cells [SMC], myofibroblasts), cell migration (SMC from the media, myofibroblasts from the periadventitia, macrophages from luminal and adventitial side, platelets from luminal side), extracellular matrix deposition, and inflammatory processes (leukocytes, platelets, growth factors, cytokines). Disrupted elastin fibers within the vein vessel wall in fibrin control veins add data to the possible detrimental effect of fibrin. This could possibly be a factor, which contributes to long-term vein graft failure. Our data raise the question whether the possible detrimental effects of local fibrin application on vein grafts contradict the theoretical advantages of drug delivery through local fibrin application. These experimental data are supported by clinical observations showing an unfavorable risk—benefit relation for the intraoperative use of fibrin glue [16, 17]
References
Veith FJ, Gupta SK, Ascer E et al (1986) Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg 3(1):104
Motwani JG, Topol EJ (1998) Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 97(9):916
Schwartz SM, deBlois D, O'Brien ER (1995) The intima. Soil for atherosclerosis and restenosis. Circ Res 77(3):445
O'Brien JE Jr, Shi Y, Fard A, Bauer T, Zalewski A, Mannion JD (1997) Wound healing around and within saphenous vein bypass grafts. J Thorac Cardiovasc Surg 114(1):38
Mehta D, George SJ, Jeremy JY et al (1998) External stenting reduces long-term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arteriovenous bypass grafting. Nat Med 4(2):235
Angelini GD, Lloyd C, Bush R, Johnson J, Newby AC (2002) An external, oversized, porous polyester stent reduces vein graft neointima formation, cholesterol concentration, and vascular cell adhesion molecule 1 expression in cholesterol-fed pigs. J Thorac Cardiovasc Surg 124(5):950
Stooker W, Niessen HW, Baidoshvili A et al (2001) Perivenous support reduces early changes in human vein grafts: studies in whole blood perfused human vein segments. J Thorac Cardiovasc Surg 121(2):290
Jeremy JY, Bulbulia R, Johnson JL et al (2004) A bioabsorbable (polyglactin), nonrestrictive, external sheath inhibits porcine saphenous vein graft thickening. J Thorac Cardiovasc Surg 127(6):1766
Stooker W, Niessen HW, Wildevuur WR et al (2002) Perivenous application of fibrin glue reduces early injury to the human saphenous vein graft wall in an ex vivo model. Eur J Cardiothorac Surg 21(2):212
Wan L, Li D, Wu Q (2006) Perivenous application of fibrin glue as external support enhanced adventitial adenovirus transfection in rabbit model. J Surg Res 135(2):312
Stooker W, Gok M, Sipkema P et al (2003) Pressure–diameter relationship in the human greater saphenous vein. Ann Thorac Surg 76(5):1533
Wan S, Arifi AA, Chan MC et al (2006) Differential, time-dependent effects of perivenous application of fibrin glue on medial thickening in porcine saphenous vein grafts. Eur J Cardiothorac Surg 29(5):742
Salzberg SP, Filsoufi F, Anyanwu A et al (2006) Increased neointimal formation after surgical vein grafting in a murine model of type 2 diabetes. Circulation 114(1 Suppl):I302
Moon MC, Molnar K, Yau L, Zahradka P (2004) Perivascular delivery of losartan with surgical fibrin glue prevents neointimal hyperplasia after arterial injury. J Vasc Surg 40(1):130
Robbie L, Libby P (2001) Inflammation and atherothrombosis. Ann N Y Acad Sci 947:167
Goerler H, Oppelt P, Abel U, Haverich A (2007) Safety of the use of Tissucol((R)) Duo S in cardiovascular surgery: retrospective analysis of 2149 patients after coronary artery bypass grafting. Eur J Cardiothorac Surg 32(4):560
Lamm P, Adelhard K, Juchem G et al (2007) Fibrin glue in coronary artery bypass grafting operations: casting out the Devil with Beelzebub? Eur J Cardiothorac Surg 32(4):567
Acknowledgments
The expert technical assistance of Mrs. Waldmann-Beushausen is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Stojanovic, T., El-Sayed Ahmad, A., Didilis, V. et al. Extravascular perivenous fibrin support leads to aneurysmal degeneration and intimal hyperplasia in arterialized vein grafts in the rat. Langenbecks Arch Surg 394, 357–362 (2009). https://doi.org/10.1007/s00423-008-0341-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0341-3