Abstract
Background
Cellular stress during reoxygenation is a common phenomenon in solid organ transplantation and is characterized by production of reactive oxygen species. Herein, we studied in isolated tubular segments of rat kidney cortex the impact of oxygen radical scavengers and an iron chelator on post-hypoxic recovery.
Methods
Tubules, suspended in Ringer’s solution containing 5 mM glycine, underwent 30 min hypoxia and 60 min reoxygenation. Untreated tubules served as controls. Hypoxia–reoxygenation injury was measured by membrane leakage, lipid peroxidation and cellular functions. In hypoxia–reoxygenated-isolated tubular segments, protective effects of different scavengers and of the iron chelator deferoxamine on hypoxia–reoxygenation injury were analyzed.
Results
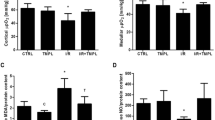
Scavengers protected isolated tubular segments from hypoxia–reoxygenation-induced cellular disintegration and dysfunction. Deferoxamine was found to exert the most distinct protection. It was further found to exert a dose-dependent protection on hypoxia–reoxygenation damage in isolated tubular segments, which was critically mediated by chelating tissue and bond iron.
Conclusions
Our data demonstrate that radical scavengers effectively protect from hypoxia–reoxygenation injury in isolated tubular segments and that the iron chelator deferoxamine is especially a potent inhibitor of iron ion-mediated hypoxia–reoxygenation damage. Thus, inclusion of this iron chelator in organ storage solutions might improve post-transplant organ function and protect from reperfusion injury.






Similar content being viewed by others
References
Gronow G, Moussavian M, Malyusz M (1999) Effect of hydroxyl radical scavengers in renal cortical cells. Adv Exp Med Biol 471:345–351
Vinas JL, Sola A, Hotter G (2006) Mitochondrial NOS upregulation during renal I/R causes apoptosis in a peroxynitrite-dependent manner. Kidney Int 69:1403–1409
Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA et al (2007) Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther 320:1050–1060
Weinberg JM, Roeser NF, Davis jA, Venkatachalam MA (1997) Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52:140–151
Gronow G, Malyusz M, Niedermayer W, Klause N (1994) Diminution of histidine-induced reoxygenation damage by glycine in posthypoxic renal cells. Adv Exp Med Biol 1345:717–722
Gronow G, Klause N, Malyusz M (1994) Restriction of hypoxic membrane defect by glycine improves mitochondrial and cellular function in reoxygenated renal tubules. Adv Exp Med Biol 361:585–589
Plin C, Tillement JP, Berdeaux A, Morin D (2005) Resveratrol protects against cold ischemia–warm reoxygenation-induced damages to mitochondria and cells in rat liver. Eur J Pharmacol 528:162–168
Zager RA, Burkhart K (1997) Myoglobin toxicity in proximal human kidney cells: roles of Fe, Ca2+, H2O2, and terminal mitochondrial electron transport. Kidney Int 51:728–738
Hashimoto T, Hirata M, Itoh T, Kanmura Y, Kuriyama H (1986) Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol 370:605–618
Gronow GH, Cohen JJ (1984) Substrate support for renal functions during hypoxia in the perfused rat kidney. Am J Physiol 247:618–631
Bertermann H, Gronow G, Weiss C (1975) An improved technique for metabolic studies on isolated cortical cells and tubules of the rat kidney. Curr Probl Clin Biochem 4:76–78
Knox CD, Pierce JM, Nicoud IB, Belous AE, Jones CM, Anderson CD et al (2006) Inhibition of phospholipase C attenuates liver mitochondrial calcium overload following cold ischemia. Transplantation 81:567–572
Cherry PD, Omar HA, Farrell KA, Stuart JS, Wolin MS (1990) Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol 259:1056–1062
Salvemini D, Doyle TM, Cuzzocrea S (2006) Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans 34:965–970
Khalid MA, Ashraf M (1992) Maximal OH production is seen upon reoxygenation of viable anoxic cultured cardiomyocytes but not of compromised cells. Am J Cardiovasc Pathol 4:245–255
Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM (2007) Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species upregulation and genetic instability. Cancer Res 67:4671–4678
Ozer MK, Parlakpinar H, Cigremis Y, Ucar M, Vardi N, Acet A (2005) Ischemia-reperfusion leads to depletion of glutathione content and augmentation of malondialdehyde production in the rat heart from overproduction of oxidants: can caffeic acid phenethyl ester (CAPE) protect the heart? Mol Cell Biochem 273:169–175
Martin LJ, Chen K, Liu Z (2005) Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci 25:6449–6459
Chen L, Lee HM, Greeley GH Jr, Englander EW (2007) Accumulation of oxidatively generated DNA damage in the brain: a mechanism of neurotoxicity. Free Radic Biol Med 42:385–393
Barondeau DP, Kassmann CJ, Tainer JA, Getzoff ED (2006) Understanding GFP posttranslational chemistry: structures of designed variants that achieve backbone fragmentation, hydrolysis, and decarboxylation. J Am Chem Soc 128:4685–4693
Zecchina A, Rivallan M, Berlier G, Lamberti C, Ricchiardi G (2007) Structure and nuclearity of active sites in Fe-zeolites: comparison with iron sites in enzymes and homogeneous catalysts. Phys Chem Chem Phys 9:3483–3499
Maiti D, Sarjeant AA, Karlin KD (2007) Copper(II)-hydroperoxo complex induced oxidative N-dealkylation chemistry. J Am Chem Soc 129:6720–6721
Droge W, Schipper HM (2007) Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 6:361–370
de Vries B, Walter SJ, von Bonsdorff L, Wolfs TG, van Heurn LW, Parkkinen J et al (2004) Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation 77:669–675
Wang XH, Wang K, Zhang F, Li XC, Li J, De W et al (2005) Heme oxygenase-1 alleviates ischemia-reperfusion injury in aged liver. World J Gastroenterol 11:690–694
Weinberg JM, Davis JA, Abarzua M, Kunkel R (1990) Protection by glycine of proximal tubules from injury due to inhibitors of mitochondrial ATP production. Am J Physiol 258:1127–1140
Paller MS, Patten M (1992) Protective effects of glutathione, glycine, or alanine in an in vitro model of renal anoxia. J Am Soc Nephrol 2:1338–1344
Moussavian MR, Slotta JE, Kollmar O, Menger MD, Schilling MK, Gronow G (2007) Hemoglobin induces cytotoxic damage of glycine-preserved renal tubules. Transpl Int 20:884–894
Nakamura J, Purvis ER, Swenberg JA (2003) Micromolar concentrations of hydrogen peroxide induce oxidative DNA lesions more efficiently than millimolar concentrations in mammalian cells. Nucleic Acids Res 31:1790–1795
Grassmann E, Reichlmayr-Lais AM, Kirchgessner M, Kim JJ (1983) Iron concentration in various organs of rats following administration of various iron and protein loads. Z Ernahrungswiss 22:195–204
Paller MS (1994) The cell biology of reperfusion injury in the kidney. J Investig Med 42:632–639
Liu M, Okada S (1994) Induction of free radicals and tumors in the kidneys of Wistar rats by ferric ethylenediamine-N,N′-diacetate. Carcinogenesis 15:2817–2821
Zhao G, Ayene IS, Fisher AB (1997) Role of iron in ischemia-reperfusion oxidative injury of rat lungs. Am J Respir Cell Mol Biol 16:293–299
Shadid M, Van Bel F, Steendijk P, Dorrepaal CA, Moison R, Van Der Velde ET, Baan J (1999) Effect of deferoxamine on post-hypoxic-ischemic reperfusion injury of the newborn lamb heart. Biol Neonate 75:239–249
Bauer C, Marzi I, Larsen R (1997) Deferoxamine-conjugated hydroxyethyl starch reduces reperfusion injury to the liver following hemorrhagic shock. Anaesthesist 46:53–56
Author information
Authors and Affiliations
Corresponding author
Additional information
German Society of Surgery, Surgical Forum 2008, Best of Abstracts.
Rights and permissions
About this article
Cite this article
Moussavian, M.R., Slotta, J.E., Kollmar, O. et al. Post-hypoxic cellular disintegration in glycine-preserved renal tubules is attenuated by hydroxyl radical scavengers and iron chelators. Langenbecks Arch Surg 393, 303–310 (2008). https://doi.org/10.1007/s00423-008-0287-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0287-5