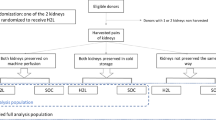
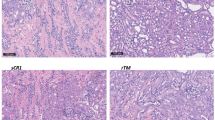
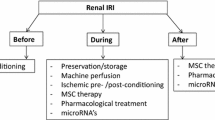
Abstract
Ischemia-reperfusion injury (IRI) is an unavoidable and unresolved problem that poses a great challenge in kidney transplantation. It represents a major factor that increases acute tubular necrosis, decreases graft survival, and delays renal graft function. This complicates graft quality, posttransplant patient care, and kidney transplantation outcomes and therefore undermines the success of kidney transplantation. In this chapter, we present recent advances in research regarding novel pharmacological strategies involving the use of hydrogen sulfide (H2S), the third established member of the gasotransmitter family, against IRI in different experimental models of involving transplantation of kidney and other transplantable solid organs. Additionally, we also discuss the molecular mechanisms underlying the effects of H2S donor molecules in transplantation and suggestions for clinical translation. Our findings in this chapter showed that storage of renal graft and other solid organ grafts in H2S-supplemented preservation solution or administration of H2S to organ donor prior to organ procurement and to recipient at the start and during reperfusion is a novel, simple, and cost-effective pharmacological approach to minimize cold IRI, limit posttransplant complications, and improve transplantation outcomes. In conclusion, experimental evidence demonstrates that H2S can significantly mitigate IRI during transplantation through inhibition of a complex cascade of interconnected cellular and molecular events involving microcirculatory disturbance and microvascular dysfunction, mitochondrial injury, inflammatory responses, cell damage and cell death, and other damaging molecular pathways while promoting protective pathways. Translating these promising findings from bench to bedside will lay the foundation for the use of H2S in clinical organ transplantation in the future.
This chapter is a modified version by the same authors in the publication titled H2S donor molecules against cold ischemia-reperfusion injury in preclinical models of solid organ transplantation. Pharmacol Res. 2021 Oct; 172:105842.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dorweiler B, Pruefer D, Andrasi TB, Maksan SM, Schmiedt W, Neufang A, Vahl CF. Ischemia-reperfusion injury. Eur J Trauma Emerg Surg. 2007;33(6):600–12.
Lobb I, Davison M, Carter D, Liu W, Haig A, Gunaratnam L, Sener A. Hydrogen sulfide treatment mitigates renal allograft ischemia-reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J Urol. 2015;194(6):1806–15.
Grewal J, Lobb I, Saha M, Haig A, Jiang J, Sener A. Mp29-16 hydrogen sulfide supplementation mitigates effects of ischemia reperfusion injury in a murine model of donation after cardiac death renal transplantation. J Urol. 2016;195(4):e386.
Wang R. Gasotransmitters: growing pains and joys. Trends Biochem Sci. 2014;39(5):227–32.
Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;6(3):1066–71.
Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114(4):730–7.
Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518.
Lobb I, Mok A, Lan Z, Liu W, Garcia B, Sener A. Supplemental hydrogen sulphide protects transplant kidney function and prolongs recipient survival after prolonged cold ischaemia-reperfusion injury by mitigating renal graft apoptosis and inflammation. Br J Urol Int. 2012;110(11c):E1187–95.
Yamamoto J, Sato W, Kosugi T, Yamamoto T, Kimura T, Taniguchi S, et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin Exp Nephrol. 2013;17(1):32–40.
Dugbartey GJ, Bouma HR, Lobb I, Sener A. Hydrogen sulfide: a novel nephroprotectant against cisplatin-induced renal toxicity. Nitric Oxide. 2016;57:15–20.
Dugbartey GJ. Diabetic nephropathy: a potential savior with ‘rotten-egg’ smell. Pharmacol Rep. 2017;69:331–9.
Dugbartey GJ. H2S as a possible therapeutic alternative for the treatment of hypertensive kidney injury. Nitric Oxide. 2017;64:52–60.
Dugbartey GJ. The smell of renal protection against chronic kidney disease: hydrogen sulfide offers a potential stinky remedy. Pharmacol Rep. 2018;70(2):196–205.
Dugbartey GJ, Bouma HR, Saha MN, Lobb I, Henning RH, Sener A. A hibernation-like state for transplantable organs: is hydrogen sulfide therapy the future of organ preservation? Antioxid Redox Signal. 2018;28(16):1503–15.
Xia M, Chen L, Muh RW, Li PL, Li N. Production and action of hydrogen sulfide, a novel gaseous bioactive substance in the kidneys. J Pharmacol Exp Ther. 2009;329:1056–62.
Mikami Y, Shinuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439:479–85.
Shibuya N, Koike S, Tanaka M, et al. A novel pathway for the production of hydrogen sulfide from d-cysteine in mammalian cells. Nat Commun. 2013;4:1366.
Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron transport flow and supports cellular biogenesis. FASEB J. 2013;27:601–11.
Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal. 2014;20(5):783–93.
Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav Immun. 2011;25:110–9.
Bao L, Vlcek C, Paces V, Kraus JP. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch Biochem Biophys. 1998;350(1):95–103.
Lee HJ, Mariappan MM, Feliers D, Cavaglieri RC, Sataranatarajan K, Abboud HE, et al. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J Biol Chem. 2012;387(7):4451–61.
Dugbartey GJ, Talaei F, Houwertjes MC, Goris M, Epema AH, Bouma HR, Henning RH. Dopamine treatment attenuates acute kidney injury in a rat model of deep hypothermia and rewarming—the role of renal H2S-producing enzymes. Eur J Pharmacol. 2015;769:225–33.
Jin S, Pu S, Hou C, Ma F, Li N, Li X, Tan B, Tao B, Wang M, Zhu Y. Cardiac H2S generation is reduced in ageing diabetic mice. Oxid Med Cell Longev. 2015;2015:758358.
Persa C, Osmotherly K, Kate C-W, Moon S, Lou MF. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a trans-sulfuration pathway for oxidative stress defense. Exp Eye Res. 2006;83(4):817–23.
Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, et al. Hydrogen sulfide as a novel nociceptive messenger. Pain. 2007;132:74–81.
Wang XB, Huang XM, Ochs T, Li XY, Jin HF, Tang CS, Du JB. Effect of sulfur dioxide preconditioning on rat myocardial ischemia/reperfusion injury by inducing endoplasmic reticulum stress. Basic Res Cardiol. 2011;106(5):865–78.
Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands JL, Ploeg RJ, Yang G, Leuvenink HG, van Goor H. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24(5):759–70.
Nagahara N, Hirasawa T, Yoshii T, Niimura Y. Is novel signal transducer sulfur oxide involved in the redox cycle of persulfide at the catalytic site cysteine in a stable reaction intermediate of mecaptopyruvate sulfurtransferase? Antioxid Redox Signal. 2012;16:747–53.
Coletta C, Módis K, Szczesny B, Brunyánszki A, Oláh G, Rios EC, Yanagi K, Ahmad A, Papapetropoulos A, Szabo C. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by dl-α-lipoic acid. Mol Med. 2015;21(1):1–14.
Tomita M, Nagahara N, Ito T. Expression of 3-mercaptopyruvate sulfurtransferase in the mouse. Molecules. 2016;21(12):1707.
Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RB, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A. 2007;104:17977–82.
Predmore BL, Kondo K, Bhushan S, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302(11):H2410–8.
Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20.
Li Q, Lancaster JR Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34.
Zhao Y, Biggs TD, Xian M. Hydrogen sulfide (H2S)-releasing agents: chemistry and biological applications. Chem Commun (Camb). 2014;50(80):11788–805.
Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem. 2010;53:6275–86.
Kashfi K, Olso KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85:689–703.
Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–60.
Le Trionairre S, Perry A, Bartosz S, et al. The synthesis and functional evaluation of mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl)triphenyl phosphonium bromide (AP39). Med Chem Commun. 2014;5:728–36.
Ahmad A, Olah G, Szczesny B, Wood ME, Whiteman M, Szabo C. AP39, a mitochondrially-targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury, in vivo. Shock. 2016;45:88–97.
Zhao FL, Fang F, Qiao PF, Yan N, Gao D, Yan Y. AP39, a mitochondria-targeted hydrogen sulfide donor, supports cellular bioenergetics and protects against Alzheimer’s disease by preserving mitochondrial function in APP/PS1 mice and neurons. Oxid Med Cell Longev. 2016;2016:8360738.
Ginter E, Simko V. Garlic (Allium sativum L.) and cardiovascular diseases. Bratisl Lek Listy. 2010;111:452–6.
Balaban CL, Rodriguez JV, Guibert EE. Delivery of the bioactive gas hydrogen sulfide during cold preservation of rat liver: effects on hepatic function in an ex vivo model. Artif Organs. 2011;35:508515.
Sun X, Wang W, Dai J, Huang J, Shi M, Chu X, Wang F, Guo C, Wang C, Pang L, Wang Y. Donor heart preservation with a novel long-term and slow-releasing hydrogen sulfide system. Nitric Oxide. 2018;81:1–10.
Marutani E, Yamada M, Ida T, et al. Thiosulfate mediates cytoprotective effects of hydrogen sulfide against neuronal ischemia. J Am Heart Assoc. 2015;4(11):e002125.
Mohan D, Balasubramanian ED, Ravindran S, Kurian GA. Renal mitochondria can withstand hypoxic/ischemic injury secondary to renal failure in uremic rats pretreated with sodium thiosulfate. Indian J Pharmacol. 2017;49(4):317–21.
Ravindran S, Boovarahan SR, Shanmugam K, Vedarathinam RC, Kurian GA. Sodium thiosulfate preconditioning ameliorates ischemia/reperfusion injury in rat hearts via reduction of oxidative stress and apoptosis. Cardiovasc Drugs Ther. 2017;31(5–6):511–24.
Snijder PM, Frenay AR, Koning AM, et al. Sodium thiosulfate attenuates angiotensin II-induced hypertension, proteinuria and renal damage. Nitric Oxide. 2014;42:87–98.
Bijarnia RK, Bachtler M, Chandak PG, van Goor H, Pasch A. Sodium thiosulfate ameliorates oxidative stress and pre-serves renal function in hyperoxaluric rats. PLoS One. 2015;10(4):e0124881.
Wallace JL, Vaughan D, Dicay M, MacNaughton WK, de Nucci G. Hydrogen sulfide-releasing therapeutics: translation to the clinic. Antioxid Redox Signal. 2018;28(16):1533–40.
Opelz G, Döhler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83(3):247–53.
Morris PJ. Kidney transplantation, principles and practice. 6th ed. Saunders; 2008. p. 126–7.
Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009;13(38):3–4.
Dragun D, Hoff U, Park JK, et al. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60(3):1173–81.
Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allo-grafts. Kidney Int. 2004;65(2):713–8.
Quiroga I, McShane P, Koo DD, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21(6):1689–96.
Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11(12):2647–56.
Hosgood SA, Nicholson ML. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br J Surg. 2010;97(2):202–9.
Lobb I, Zhu J, Liu W, Haig A, Lan Z, Sener A. Hydrogen sulfide treatment improves long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can Urol Assoc J. 2014;8(5–6):413.
Lobb I, Jiang J, Lian D, Liu W, Haig A, Saha MN, Torregrossa R, Wood ME, Whiteman M, Sener A. Hydrogen sulfide protects renal grafts against prolonged cold ischemia-reperfusion injury via specific mitochondrial actions. Am J Transplant. 2017;17(2):341–52.
Gero D, Szabo C. Glucocorticoids suppress mitochondrial oxidant production via upregulation of uncoupling protein 2 in hyperglycemic endothelial cells. PLoS One. 2016;11(4):e0144813.
Suzuki K, Olah G, Modis K, Colleta C, Kulp G, Gero D, et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 2011;108(33):13829–34.
Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands JL, Ploeg RJ, Yang G, Leuvenink HG, van Goor H. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24(5):759–70.
Zhu C, Su Y, Juriasingani S, Zheng H, Veramkovich V, Jiang J, Sener A, Whiteman M, Lacefield J, Nagpal D, Alotaibi F, Liu K, Zheng X. Supplementing preservation solution with mitochondria-targeted H2S donor AP39 protects cardiac grafts from prolonged cold ischemia-reperfusion injury in heart transplantation. Am J Transplant. 2019;19(11):3139–48.
Strutynska NA, Dorofeieva NO, Vavilova HL, Sahach VF. Hydrogen sulfide inhibits Ca2+-induced mitochondrial transition pore opening in spontaneously hypertensive rats. Fiziol Zh. 2013;59:310.
Chatzianastasiou A, Bibli SI, Andreadou I, Efentakis P, Kaludercic N, Wood ME, Whiteman M, Di Lisa F, Daiber A, Manolopoulos VG, Szabó C, Papapetropoulos A. Cardioprotection by H2S donors: nitric oxide-dependent- and independent mechanisms. J Pharmacol Exp Ther. 2016;358:43140.
Karwi QG, Bornbaum J, Boengler K, Torregrossa R, Whiteman M, Wood ME, Schulz R, Baxter GF. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br J Pharmacol. 2017;174:287301.
Brinkkoetter PT, Song H, Lösel R, Schnetzke U, Gottmann U, Feng Y, Hanusch C, Beck GC, Schnuelle P, Wehling M, van der Woude FJ, Yard BA. Hypothermic injury: the mitochondrial calcium, ATP and ROS love–hate triangle out of balance. Cell Physiol Biochem. 2008;22:195204.
Calvert JW, Jha S, Gundewar S, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–74.
Shimada S, Fukai M, Wakayama K, et al. Hydrogen sulfide augments survival signals in warm ischemia and reperfusion of the mouse liver. Surg Today. 2015;45:892–903.
Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486:47–58.
Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–16.
Pareira de Avila MA, Giusti-Paiva A, de Oliveira G, Nascimento C. The peripheral antinociceptive effect induced by the heme oxygenase/carbon monoxide pathway is associated with ATP-sensitive K+ channels. Eur J Pharmacol. 2014;726:41–8.
Wu J, Wei J, You X, Chen X, Zhu H, Zhu X, Liu Y, Xu M. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J Surg Res. 2013;182:e2533.
George TJ, Arnaoutakis GJ, Beaty CA, Jandu SK, Santhanam L, Berkowitz DE, Shah AS. Hydrogen sulfide decreases reactive oxygen in a model of lung transplantation. J Surg Res. 2012;178:494501.
George TJ, Arnaoutakis GJ, Beaty CA, Jandu SK, Santhanam L, Berkowitz DE, Shah AS. Inhaled hydrogen sulfide improves graft function in an experimental model of lung transplantation. J Surg Res. 2012;178:593600.
Jiang T, Yang W, Zhang H, Song Z, Liu T, Lv X. Hydrogen sulfide ameliorates lung ischemia-reperfusion injury through sirt1 signaling pathway in type 2 diabetic rats. Front Physiol. 2020;11:596.
Haigis MC, Guarente LP. Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21.
Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–75.
Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxidative Med Cell Longev. 2017;2017:7543973.
Balaban CL, Rodriguez JV, Tiribelli C, Guibert EE. The effect of a hydrogen sulfide releasing molecule (Na2S) on the cold storage of livers from cardiac dead donor rats. A study in an ex vivo model. Cryobiology. 2015;71:2432.
Prudhomme T, Kervella D, Le Bas-Bernardet S, Cantarovich D, Karam G, Blancho G, Branchereau J. Ex situ perfusion of pancreas for whole-organ transplantation: is it safe and feasible? A systematic review. J Diabetes Sci Technol. 2020;14(1):120–34.
Nishime K, Miyagi-Shiohira C, Kuwae K, Tamaki Y, Yonaha T, Sakai-Yonaha M, Saitoh I, Watanabe M, Noguchi H. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am J Transplant. 2021;21(8):2698–708.
Iyer KR, Kunecki M, Boullata JI, Fujioka K, Joly F, Gabe S, Pape UF, Schneider SM, Virgili Casas MN, Ziegler TR, Li B, Youssef NN, Jeppesen PB. Independence from parenteral nutrition and intravenous fluid support during treatment with teduglutide among patients with intestinal failure associated with short bowel syndrome. JPEN J Parent Enteral Nutr. 2017;41(6):946–51.
Celik N, Mazariegos GV, Soltys K, Rudolph JA, Shi Y, Bond GJ, Sindhi R, Ganoza A. Pediatric intestinal transplantation. Gastroenterol Clin N Am. 2018;47(2):355–68.
Guo M, Lu C, Gao Y, Zhang H, Chen D, Li Y. Lifor solution: an alternative preservation solution in small bowel transplantation. Gastroenterol Res Pract. 2016;2016:3925751.
Lautenschläger I, Pless-Petig G, Middel P, de Groot H, Rauen U, Stojanovic T. Cold storage injury to rat small-bowel transplants-beneficial effect of a modified HTK solution. Transplantation. 2018;102(10):1666–73.
Lysyy T, Finotti M, Maina RM, Morotti R, Munoz-Abraham AS, Bertacco A, Ibarra C, Barahona M, Agarwal R, D'Amico F, Rodriguez-Davalos MI, Mulligan D, Geibel J. Human small intestine transplantation: segmental susceptibility to ischemia using different preservation solutions and conditions. Transplant Proc. 2020;52(10):2934–40.
Zaman J, Chakma A. Production of hydrogen and sulfur from hydrogen sulfide. Fuel Process Technol. 1995;41(2):159–98.
Buchholz BM, Masutani K, Kawamura T, Peng X, Toyoda Y, Billiar TR, Bauer AJ, Nakao A. Hydrogen-enriched preservation protects the isogeneic intestinal graft and amends recipient gastric function during transplantation. Transplantation. 2011;92(9):985–92.
Henderson PW, Weinstein AL, Sohn AM, Jimenez N, Krijgh DD, Spector JA. Hydrogen sulfide attenuates intestinal ischemia-reperfusion injury when delivered in the post-ischemic period. J Gastroenterol Hepatol. 2010;25(10):1642–7.
Liu H, Bai X, Shi S, Cao Y. Hydrogen sulfide protects from intestinal ischaemia-reperfusion injury in rats. J Pharm Pharmacol. 2009;61(2):207–12.
Cui N, Luo H, Zhao Y. Protective effect of GYY4137, a water-soluble hydrogen sulfide-releasing molecule, on intestinal ischemia-reperfusion. Mol Med Rep. 2020;21(3):1633–9.
Jensen AR, Drucker NA, Khaneki S, Ferkowicz MJ, Markel TA. Hydrogen sulfide improves intestinal recovery following ischemia by endothelial nitric oxide-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G450–6.
Drucker NA, Jensen AR, Te Winkel JP, Markel TA. Hydrogen sulfide donor GYY4137 acts through endothelial nitric oxide to protect intestine in murine models of necrotizing enterocolitis and intestinal ischemia. J Surg Res. 2019;234:294–302.
Pfeifle CE, Howell SB, Felthouse RD, Woliver TB, Andrews PA, Markman M, Murphy MP. High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol. 1985;3(2):237–44.
Breen PH, Isserles SA, Westley J, Roizen MF, Taitelman UZ. Effect of oxygen and sodium thiosulfate during combined carbon monoxide and cyanide poisoning. Toxicol Appl Pharmacol. 1995;134(2):229–34.
Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149(8):946–9.
Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(1):63–74.
Bucci M, Vellecco V, Cantalupo A, Brancaleone V, Zhou Z, Evangelista S, et al. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovasc Res. 2014;102:138–47.
Zhang MY, Dugbartey GJ, Juriasingani S, Akbari M, Liu W, Haig A, McLeod P, Arp J, Sener A. Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed Pharmacother. 2022;145:112435.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
None.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dugbartey, G.J., Sener, A. (2023). Hydrogen Sulfide Against Ischemia-Reperfusion Injury in Transplantation of Kidney and Other Transplantable Solid Organs. In: Hydrogen Sulfide in Kidney Diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-44041-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-44041-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44040-3
Online ISBN: 978-3-031-44041-0
eBook Packages: MedicineMedicine (R0)