Abstract
Background
After flap surgery, vasomotion, defined as oscillation of the arteriolar diameter, may protect tissue during critical perfusion conditions. The mechanisms that regulate vasomotion are still unclear; therefore, we studied the incidence of vasomotion in peripheral tissue and whether nitric oxide or endothelins are involved in regulation of vasomotion.
Materials and methods
In Sprague–Dawley rats, an osteomyocutaneous flap was prepared. To induce critical perfusion conditions, we reduced arterial blood flow supplying the flap to 0.15 ml/min. Seven animals received NG-nitro-l-arginine methyl ester (L-NAME), a nitric oxide-synthase inhibitor, and six animals bosentan, an endothelin A/B receptor antagonist. Microcirculation of muscle, skin, subcutis and periosteum was assessed by intravital microscopy before and after drug application.
Results
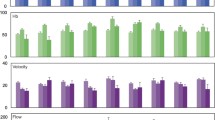
In all animals, reduction of arterial blood supply induced arteriolar vasomotion in muscle (100%), but not in periosteum, subcutis and skin. Vasomotion was found to be affected by neither L-NAME (frequency 2.6±0.2 versus 2.4±0.2 cycles/min; amplitude 67±19 versus 55±20%; share of dilation period in vasomotion cycle 59±2 versus 58±3%) nor bosentan (1.8±0.1 versus 1.7±0.1 cycles/min; 60±10 versus 64±6%; 50±2 versus 53±1%).
Conclusions
Our study indicates that during critical perfusion conditions, arteriolar vasomotion develops only in muscle, not in skin, subcutis and periosteum, and that nitric oxide and endothelins are not involved in the regulation of this protective vascular response.




Similar content being viewed by others
References
Rücker M, Vollmar B, Roesken F, Spitzer WJ, Menger MD (2002) Microvascular transfer-related abrogation of capillary flow motion in critically reperfused composite flaps. Br J Plast Surg 55:129–135
Allegra C, Intaglietta M, Messmer K (1989) Vasomotion and flowmotion. Prog Appl Microcirc 20:1–88
Rücker M, Strobel O, Vollmar B, Roesken F, Menger MD (2000) Vasomotion in critically perfused skeletal muscle protects adjacent tissues from capillary perfusion failure. Am J Physiol 279:H550–H558
Schmidt JA, Borgström P, Intaglietta M (1993) The vascular origin of slow wave flowmotion in skeletal muscle during local hypotension. Int J Microcirc Clin Exp 12:287–297
Lamontagne D, Pohl U, Busse R (1992) Mechanical deformation of vessel wall and shear stress determine the basal release of endothelium-derived relaxing factor in the intact rabbit coronary vascular bed. Circ Res 70:123–130
Marsault R, Vigne P, Breittmayer JP, Frelin C (1991) Kinetics of vasoconstrictor action of endothelins. Am J Physiol 261:C986–C993
Toribatake Y, Tomita K, Kawahara N, Baba H, Ohnari H, Tanaka S (1997) Regulation of vasomotion of arterioles and capillaries in the cat spinal cord: role of alpha actin and endothelin-1. Spinal Cord 35:26–32
Rücker M, Roesken F, Schäfer T, Spitzer WJ, Vollmar B, Menger MD (1999) In vivo analysis of the microcirculation of osteomyocutaneous flaps using fluorescence microscopy. Br J Plast Surg 52:644–652
Rücker M, Schäfer T, Roesken F, Spitzer WJ, Bauer M, Menger MD (2001) Local heat-shock priming-induced improvement in microvascular perfusion in osteomyocutaneous flaps is mediated by heat-shock protein 32. Br J Surg 88:450–457
Bryant CE, Allcock GH, Warner TD (1995) Comparison of effects of chronic and acute administration of NG-nitro-l-arginine methyl ester to the rat on inhibition of nitric oxide-mediated responses. Br J Pharmacol 114:1673–1679
Clozel M, Breu V, Gray GA, Kalina B, Löffler B, Burri K, Cassal J, Hirth G, Müller M, Neidhart W, Ramuz H (1994) Pharmacological characterization of Bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther 270:228–235
Vollmar B, Preissler G, Menger MD (1994) Hemorrhagic hypotension induces arteriolar vasomotion and intermittent capillary perfusion in rat pancreas. Am J Physiol 267:H1936–H1940
Tsai, AG, Intaglietta M (1993) Evidence of flowmotion induced changes in local tissue oxygenation. Int J Microcirc Clin Exp 12:75–88
Borgström P, Bruttig B, Lindbom L, Intaglietta M, Arfors KE (1990) Microvascular responses in rabbit skeletal muscle after fixed volume hemorrhage. Am J Physiol 259:H190–H196
Erni D, Banic A, Wheatley AM, Sigurdsson GH (1995) Haemorrhage during anaesthesia and surgery: continuous measurement of microcirculatory blood flow in the kidney, liver, skin, and skeletal muscle. Eur J Anaesthesiol 12:423–429
Borgström P, Schmidt JA, Bruttig SP, Intaglietta M, Arfors KE (1992) Slow-wave flowmotion in rabbit skeletal muscle after acute fixed-volume hemorrhage. Circ Shock 36:57–61
Colantuoni A, Bertuglia S, Intaglietta M (1984) Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol 246:H508–H517
Weiner RM, Borgström P, Intaglietta M (1989) Induction of vasomotion by hemorrhagic hypotension in rabbit tenuissimus muscle. Prog Appl Microcirc 15:93–99
Colantuoni A, Bertuglia S, Intaglietta M (1994) Microvascular vasomotion: origin of laser Doppler flux motion. Int J Microcirc Clin Exp 14:151–158
Bouskela E, Grampp W (1992) Spontaneous vasomotion in hamster cheek pouch arterioles in varying experimental conditions. Am J Physiol 262:H478–H485
Jackson WF, Mülsch A, Busse R (1991) Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am J Physiol 260:H248–H253
Goligorsky MS, Colflesh D, Gordienko D, Moore LC. (1995) Branching points of renal resistance arteries are enriched in L-type calcium channels and initiate vasoconstriction. Am J Physiol 268:F251–F257
Bassenge E (1995) Control of coronary blood flow by autocoids. Basic Res Cardiol 90:125–141
Bertuglia S, Colantuoni A, Intaglietta M (1994) Effects of L-NMMA and indomethacin on arteriolar vasomotion in skeletal muscle microcirculation of conscious and anesthetized hamsters. Microvasc Res 48:68–84
Buerk DG, Riva CE (1998) Vasomotion and spontaneous low-frequency oscillations in blood flow and nitric oxide in cat optic nerve head. Microvasc Res 55:103–112
Acknowledgement
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Me 900/1–3 and 1–4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of the material was presented at the International Symposium "Significance of Musculo-Skeletal Soft Tissue on Pre-Operative Planning, Surgery and Healing", 13–14 February 2003, Berlin, Germany
Rights and permissions
About this article
Cite this article
Rücker, M., Strobel, O., Vollmar, B. et al. Protective skeletal muscle arteriolar vasomotion during critical perfusion conditions of osteomyocutaneous flaps is not mediated by nitric oxide and endothelins. Langenbecks Arch Surg 388, 339–343 (2003). https://doi.org/10.1007/s00423-003-0389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-003-0389-z