Abstract
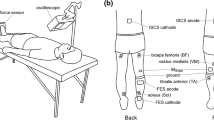
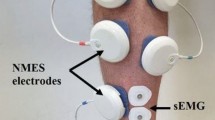
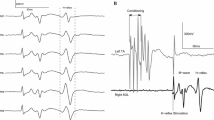
The purpose of this study was to determine to what extent one session of isotonic and isometric ankle dorsi and plantar flexion training induces changes in the frequency-dependent depression of the soleus H-reflex. Further, adaptation of reciprocal Ia inhibition exerted from tibialis anterior flexor group I afferents on soleus motoneurons, and presynaptic inhibition of Ia afferent terminals induced by a conditioning afferent volley following stimulation of the antagonist nerve were established with subjects seated before and after training. The soleus H-reflexes evoked at the inter-stimulus intervals of 1, 2, 3, 5, and 8 s were normalized to the mean amplitude of the H-reflex evoked every 10 s. Conditioned H-reflexes were normalized to the associated control H-reflex evoked with subjects seated before and after training. Twenty-six subjects were randomly assigned to one or more of the 4 exercise groups. Isometric ankle dorsi flexion training decreased the reciprocal and presynaptic inhibition, while isotonic ankle dorsi flexion had no significant effects. Isotonic plantar flexion training decreased only the reciprocal inhibition, whilst isometric plantar flexion had no significant effects on the reciprocal or presynaptic inhibition. None of the training exercise protocols affected the amount of homosynaptic depression of the soleus H-reflex. Our findings support the notion that plastic changes of reciprocal and presynaptic inhibition due to exercise are transferrable to a resting state, and that homosynaptic depression remains unaltered after a single session of ankle training. Further research is needed to outline the time-course of plastic changes of spinal inhibitory mechanisms in humans.





Similar content being viewed by others
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318
Adkins DL, Boychuk J, Remple MS, Klelm JA (2006) Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol 101:1776–1782
Baldissera F, Cavallari P, Fournier E, Pierrot-Deseilligny E, Shindo M (1987) Evidence for mutual inhibition of opposite Ia interneurones in the human upper limb. Exp Brain Res 66:106–114
Boorman GI, Hoffer JA, Kallesoe K, Viberg D, Mah C (1996) A measure of peripheral nerve stimulation efficacy applicable to H-reflex studies. Can J Neurol Sci 23:264–270
Brody DL, Yue DT (2000) Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci 20:2480–2494
Capaday C, Stein RB (1987) Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol Lond 392:513–522
Capaday C, Lavoie BA, Comeau F (1995) Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Can J Physiol Pharmacol 73:436–449
Carroll TJ, Riek S, Carson RG (2002) The sites of neural adaptation induced by resistance training in humans. J Physiol Lond 544:641–652
Chen G, Harata NC, Tsien RW (2004) Paired-pulse depression of unitary quantal amplitude at single hippocampal synapses. Proc Natl Acad Sci USA 101:1063–1068
Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123
Crone C, Nielsen J (1989) Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol Lond 416:255–272
Crone C, Nielsen J (1994) Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand 152:351–363
Crone C, Hultborn H, Jespersen B (1985) Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res 59:418–422
Crone C, Hultborn H, Jespersen B, Nielsen J (1987) Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol Lond 389:163–185
Delwaide PJ, Pepin JL (1991) The influence of contralateral primary afferents on Ia inhibitory interneurons in humans. J Physiol 439:161–179
Delwaide PJ, Sabatini M, Pepin JL, La Grutta V (1988) Reinforcement of reciprocal inhibition by contralateral movements in man. Exp Neurol 99:10–16
Duclay J, Martin A, Robbe A, Pousson M (2008) Spinal reflex plasticity during maximal dynamic contractions after eccentric training. Med Sci Sports Exerc 40:722–734
Eccles JC, Fatt P, Landgren S (1956) Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol 19:75–98
Faist M, Dietz V, Pierrot-Deseilligny E (1996) Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res 109:441–449
Geertsen SS, Lundbye-Jensen J, Nielsen JB (2008) Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol 105:915–922
Harrison PJ, Zytnicki D (1984) Crossed actions of group I muscle afferents in the cat. J Physiol 356:263–273
Holterman A, Roeleved K, Engstrom M, Sand T (2007) Enhanced H-reflex with resistance training is related to increased rate of force development. Eur J Appl Phys 101:301–312
Hongo T, Jankowska E, Lundberg A (1969) The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res 7:365–391
Hsu SF, Augustine GJ, Jackson MB (1996) Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron 17:501–512
Hultborn H, Jankowska E, Lindstrom S (1971) Recurrent inhibition from motor axon collateral of transmission in the Ia inhibitory pathway to motoneurones. J Physiol Lond 215:591–612
Hultborn H, Illert M, Santini M (1976a) Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. II. Effects from segmental flexor reflex pathways. Acta Physiol Scand 96:351–367
Hultborn H, Illert M, Santini M (1976b) Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. I. Disynaptic Ia inhibition of Ia inhibitory interneurones. Acta Physiol Scand 96:193–201
Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H (1996) On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108:450–462
Iles JF (1996) Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol Lond 491:197–207
Jankowska E (1992) Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38:335–378
Katz R, Meunier S, Pierrot-Deseilligny E (1988) Changes in presynaptic inhibition of Ia fibres in man while standing. Brain 111:417–437
Knikou M (2005) Effects of hip joint angle changes on intersegmental spinal coupling in human spinal cord injury. Exp Brain Res 167:381–393
Knikou M (2008) The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171:1–12
Knikou M (2011) Soleus H-reflex phase-dependent modulation during one-legged foot reaching and withdrawal in standing humans. Neurosci Lett 487:305–309
Knikou M, Mummidisetty CK (2011) Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol 231:104–112
Knikou M, Rymer WZ (2002) Effects of changes in hip joint angle on H-reflex excitability in humans. Exp Brain Res 143:149–159
Knikou M, Taglianetti C (2006) On the methods employed to record and measure the human soleus H-reflex. Somatosens Motor Res 23:55–62
Kohn A, Floeter MK, Hallett M (1997) Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res 116:375–380
Kolosova EV, Slivko EI (2006) Fatigue-induced modulation of the H reflex of soleus muscle in humans. Neurophysiology 38:360–364
Kuno M (1964) Quantal components of excitatory synaptic potentials in spinal motoneurones. J Physiol Lond 175:81–99
Llewellyn M, Yang JF, Prochazka A (1990) Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83:22–28
Lundberg A, Voorhoeve P (1962) Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand 56:201–219
Mazzocchio R, Kitago T, Liuzzi G, Wolpaw JR, Cohen LG (2006) Plastic changes in the human H-reflex pathway at rest following skillful cycling training. Clin Neurophysiol 117:1682–1691
Meunier S, Pierrot-Deseilligny E (1998) Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119:415–426
Meunier S, Kwon J, Russmann H, Ravindran S, Mazzocchio R, Cohen L (2007) Spinal use-dependent plasticity of synaptic transmission in humans after a single cycling session. J Physiol Lond 597:375–388
Morin C, Katz R, Mazieres L, Pierrot-Deseilligny E (1982) Comparison of soleus H reflex facilitation at the onset of soleus contractions produced voluntarily and during the stance phase of human gait. Neurosci Lett 33:47–53
Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB (2001) Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 124:826–837
Nielsen J, Kagamihara Y (1992) The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol Lond 456:373–391
Nielsen J, Kagamihara Y (1993) The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol Lond 464:575–593
Nielsen J, Kagamihara Y, Crone C, Hultborn H (1992) Central facilitation of Ia inhibition during tonic ankle dorsiflexion revealed after blockade of peripheral feedback. Exp Brain Res 88:651–656
Nielsen J, Crone C, Hultborn H (1993) H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol 66:116–121
Perez MA, Field-Fote EC, Floetter MK (2003) Patterned sensory stimulation induces plasticity in reciprocal Ia inhibition in humans. J Neurosci 23:2014–2018
Perez MA, Lungholt BKS, Nielsen JB (2005) Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol Lond 568:343–354
Perez MA, Lundbye-Jensen J, Nielsen JB (2007) Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol 98:3677–3687
Petersen N, Morita H, Nielsen J (1998) Evaluation of reciprocal inhibition of the soleus H-reflex during tonic plantar flexion in man. J Neurosci Methods 84:1–8
Plautz EJ, Milliken GW, Nudo RJ (2000) Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem 74:27–55
Raoul S, Roulades V, Deligny C, Leduc D, Lamy J-C, Lackmy-Vallee A, N’Guyen J-P, Damier P, Katz R (2012) Subthalamic nucleus stimulation reverses spinal motoneuron activity in parkinsonian patients. Brain 135:139–147
Rudomin P, Schmidt RF (1999) Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129:1–37
Schneider C, Capaday C (2003) Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol 89:648–656
Trimble MH, Koceja DM (1994) Modulation of the triceps surae H-reflex with training. Int J Neurosci 76:293–303
Ung R-V, Imbeault M-A, Ethier C, Brizzi L, Capaday C (2005) On the potential role of the corticospinal tract in the control and progressive adaptation of the soleus H-reflex during backward walking. J Neurophysiol 94:1133–1142
Voigt M, Chelli F, Frigo C (1998) Changes in the excitability of soleus muscle short latency stretch reflexes during human hopping after 4 weeks of hopping training. Eur J Appl Physiol 78:522–532
Wolpaw JR (2007) Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol 189:155–169
Wolpaw JR (2010) What can the spinal cord teach us about learning and memory? Neuroscientist 16:532–549
Wolpaw JR, O’Keefe JA (1984) Adaptive plasticity in the primate spinal stretch reflex: evidence for a two-phase process. J Neurosci 4:2718–2724
Wolpaw JR, Tennissen A (2001) Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 24:807–843
Acknowledgments
Authors thank all participants for their voluntary participation to the study. This work was supported in part by the New York State Department of Health (NYSDOH)/Contract No. C023690, Wadsworth Center, NY, USA. The funding source had no involvement in study design, collection, analysis, or data interpretation.
Conflict of interest
The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fausto Baldissera.
Rights and permissions
About this article
Cite this article
Jessop, T., DePaola, A., Casaletto, L. et al. Short-term plasticity of human spinal inhibitory circuits after isometric and isotonic ankle training. Eur J Appl Physiol 113, 273–284 (2013). https://doi.org/10.1007/s00421-012-2438-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2438-1