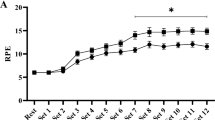
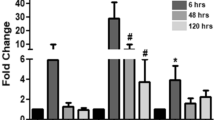
Abstract
To evaluate change in myostatin, follistatin, MyoD and SGT mRNA gene expression using eccentric exercise to study mechanisms of skeletal muscle hypertrophy. Young (28 ± 5 years) and older (68 ± 6 years) men participated in a bout of maximal single-leg eccentric knee extension on an isokinetic dynamometer at 60°/s: six sets, 12–16 maximal eccentric repetitions. Muscle biopsies of the vastus lateralis were obtained from the dominant leg before exercise and 24 h after exercise. Paired t tests were used to compare change (pre versus post-exercise) for normalized gene expression in all variables. Independent t tests were performed to test group differences (young vs. older). A probability level of P ≤ 0.05 was used to determine statistical significance with Bonferroni adjustments. We observed no significant change in myostatin (−0.59 ± 2.1 arbitrary units (AU); P = 0.42), follistatin (0.22 ± 3.4; P = 0.85), MyoD (0.23 ± 3.1; P = 0.82), or SGT (1.2 ± 6.4; P = 0.58) mRNA expression in young subjects 24 h after eccentric exercise. Similarly, we did not observe significant change in myostatin (−3.83 ± 8.8; P = 0.23), follistatin (−2.66 ± 5.2; P = 0.17), MyoD (−0.13 ± 3.1; P = 0.90), or SGT (−1.6 ± 3.5; P = 0.19) mRNA expression in older subjects. Furthermore, the non-significant changes in mRNA expression were not different between young and older subjects, P > 0.23 for all variables. Our data suggests that a single bout of maximal eccentric exercise does not alter myostatin, follistatin, MyoD or SGT mRNA gene expression in young or older subjects.

Similar content being viewed by others
References
Adams GR (1998) Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev 26:31–60
Adams GR (2006) Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab (Physiologie appliquee, nutrition et metabolisme) 31:782–790
Barber RD, Harmer DW, Coleman RA, Clark BJ (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 21:389–395
Carson JA, Alway SE (1996) Stretch overload-induced satellite cell activation in slow tonic muscle from adult and aged Japanese quail. Am J Physiol 270:C578–C584
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81:S52–S69
Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA (2006) Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab 290:E849–E855
Conboy IM, Conboy MJ, Smythe GM, Rando TA (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302:1575–1577
Dominique JE, Gerard C (2006) Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 312:2401–2414
Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA (2006) Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33:242–253
Evans WJ (1992) Exercise, nutrition and aging. J Nutr 122:796–801
Evans WJ (2002) Effects of exercise on senescent muscle. Clin Orthop Relat Res S211–S220
Evans WJ (2004) Protein nutrition, exercise and aging. J Am Coll Nutr 23:601S–609S
Evans WJ, Cannon JG (1991) The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev 19:99–125
Grounds MD (1998) Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci 854:78–91
Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD (2003) Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254
Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, Goldspink G (2004) The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol 555:231–240
Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y (2002) The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277:40735–40741
Hortobagyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG (1996) Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol 80:765–772
Jacobs SC, Wokke JH, Bar PR, Bootsma AL (1995) Satellite cell activation after muscle damage in young and adult rats. Anat Rec 242:329–336
Jemiolo B, Trappe S (2004) Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320:1043–1050
Kim JS, Cross JM, Bamman MM (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288:E1110–E1119
Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM (2006) Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101:531–544
Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA (2004) Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics 18:226–231
Marx JO, Kraemer WJ, Nindl BC, Larsson L (2002) Effects of aging on human skeletal muscle myosin heavy-chain mRNA content and protein isoform expression. J Gerontol A Biol Sci Med Sci 57:B232–B238
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147
Pilegaard H, Ordway GA, Saltin B, Neufer PD (2000) Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279:E806–E814
Psilander N, Damsgaard R, Pilegaard H (2003) Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol 95:1038–1044
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101:53–59
Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA (2003) Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 228:706–709
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Schultz E, Jaryszak DL, Valliere CR (1985) Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 8:217–222
Tamaki T, Uchiyama S, Uchiyama Y, Akatsuka A, Yoshimura S, Roy RR, Edgerton VR (2000) Limited myogenic response to a single bout of weight-lifting exercise in old rats. Am J Physiol Cell Physiol 278:C1143–C1152
Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH Jr, Kull FC Jr, Gonzalez-Cadavid N (2001) Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab 280:E221–E228
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Touchberry CD, Wacker MJ, Richmond SR, Whitman SA, Godard MP (2006) Age-related changes in relative expression of real-time PCR housekeeping genes in human skeletal muscle. J Biomol Tech 17:157–162
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Walker KS, Kambadur R, Sharma M, Smith HK (2004) Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc 36:787–793
Wang H, Zhang Q, Zhu D (2003) hSGT interacts with the N-terminal region of myostatin. Biochem Biophys Res Commun 311:877–883
Wehling M, Cai B, Tidball JG (2000) Modulation of myostatin expression during modified muscle use. FASEB J 14:103–110
Willoughby D (2004a) Effects of concentric and eccentric muscle actions on serum myostatin and follistatin-like related gene levels. J Sports Sci Med 3:226–233
Willoughby DS (2004b) Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc 36:574–582
Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98:1745–1752
Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF (2002) Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging 6:343–348
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jensky, N.E., Sims, J.K., Rice, J.C. et al. The influence of eccentric exercise on mRNA expression of skeletal muscle regulators. Eur J Appl Physiol 101, 473–480 (2007). https://doi.org/10.1007/s00421-007-0521-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0521-9