Abstract
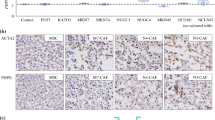
Cancer-associated fibroblasts are bioactive elements influencing the biological properties of malignant tumors. Their origin from different cell types has been established, and the possibility of their formation by epithelial-to-mesenchymal transition from cancer cells is under debate. This study shows that human cancer cells grafted to nu/nu mice induced formation of tumor stroma with the presence of typical smooth muscle actin-containing cancer-associated fibroblasts. These cells seem to be of the host origin because they are not recognized by an antibody specific for human vimentin, as was also verified in vitro. These results suggest that cancer-associated stromal fibroblasts are not formed by epithelial-to-mesenchymal transition from cancer cells.



Similar content being viewed by others
References
Augsten M (2014) Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. doi:10.3389/fonc.2014.00062. eCollection 2014
Barcellos-Hoff MH, Ravani SA (2000) Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiatede epithelial cells. Cancer Res 60:1254–1260
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Dvořánková B, Szabo P, Lacina L, Gal P, Uhrova J, Zima T, Kaltner H, André S, Gabius H-J, Syková E, Smetana K Jr (2011) Human galectins induce conversion of dermal fibroblasts into myofibroblasts and production of extracellular matrix: potential application in tissue engineering and wound repair. Cells Tissues Organs 194:469–480
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901
Fukino K, Shen L, Patocs A, Mutter GL, Eng C (2007) Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA 297:2103–2111
Gál P, Vasilenko T, Kostelníková M, Jakubco J, Kovác I, Sabol F, André S, Kaltner H, Gabius H-J, Smetana K Jr (2011) Open wound healing in vivo: monitoring, binding and the presence of adhesion/growth-regulatory galectins in rat skin during the course of complete re-epithelialization. Acta HistochemCytochem 44:191–199
Guarino M, Ab Tosoni, Nebuloni M (2009) Direct contribution of epithelium to organ fibrosis: epithelial–mesenchymal transition. Human Pathol 40:1365–1376
Hill R, Song Y, Cardiff RD, Van Dyke T (2005) Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 123:1001–1011
Hinz B (2007) Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 127:526–537
Iwasaki H, Isayama T, Ichiki T, Kikuchi M (1987) Intermediate filaments of myofibroblasts: immunochemical and immunocytochemical analyses. Pathol Res Pract 182:248–254
Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song Y-H (2011) Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell Onc 34:55–67
Kabashima-Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M, Hamamoto Y, Kawachi S, Aiura K, Kitagawa Y, Sakamoto M, Hibi T (2013) Mesenchymal stem cells regulate epithelial–mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci 104:157–164
Klíma J, Lacina L, Dvořánková B, Herrmann D, Carnwath JW, Niemann H, Kaltner H, André S, Motlík J, Gabius H-J, Smetana K Jr (2009) Differential regulation of galectin expression/reactivity during wound healing in porcine skin and in cultures of epidermal cells with functional impact on migration. Physiol Res 58:873–884
Kolář M, Szabo P, Dvořánková B, Lacina L, Gabius H-J, Strnad H, Sáchová J, Vlček C, Plzák J, Chovanec M, Cada Z, Betka J, Fík Z, Pačes J, Kovářová H, Motlík J, Jarkovská K, Smetana K Jr (2012) Upregulation of IL-6, IL-8 and CXCL-1 production in dermal fibroblasts by normal/malignant epithelial cells in vitro, immunohistochemical and transcriptomic analyses. Biol Cell 104:738–751
Kopantzev EP, Vayshlya NA, Kopantseva MR, Egorov VI, Pikunov M, Zinovyeva1 MV, Vinogradova TV, Zborovskaya IB, Sverdlov ED (2010) Cellular and molecular phenotypes of proliferating stromal cells from human carcinomas. Br J Cancer 102:1533–1540
Kundu JK, Surh YJ (2008) Inflammation: gearing the journey to cancer. Mutat Res 659:15–30
Kurose K, Hoshaw-Woodard S, Adeyinka A, Lemeshow S, Watson PH, Eng C (2001) Genetic model of multi-step breast cancerogenesis involving the epithelium and stroma: clues to tumor–microenvironment interactions. Hum Mol Genet 10:1907–1913
Lacina L, Dvořánkova B, Smetana K Jr, Chovanec M, Plzák J, Tachezy R, Kideryová L, Kučerová L, Čada Z, Bouček J, Kodet R, André S, Gabius H-J (2007) Marker profiling of normal keratinocytes identifies the stroma from squamous cell carcinoma of the oral cavity as a modulatory microenvironment in co-culture. Int Radiat Biol 83:837–848
LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19:1047–1053
López-Novoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1:303–314
McDonald LT, LaRue AC (2012) Hematopoietic stem cell derived carcinoma-associated fibroblasts: a novel origin. Int J Clin Exp Pathol 5:863–873
Mueller L, Goumas FA, Affeldt M, Sandtner S, Gehling UM, Brilloff S, Walter J, Karnatz N, Lamszus K, Rogiers X, Broering DC (2007) Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol 171:1608–1618
Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M (2014) Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys 548:20–37
Ohbayashi M, Kubota S, Kawase A, Kohyama N, Kobayashi Y, Yamamoto T (2014) Involvement of epithelial–mesenchymal transition in methotrexate-induced pulmonary fibrosis. J Toxicol Sci 39:319–330
Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Rønnov-Jessen L (2003) Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol 162:391–402
Plzák J, Lacina L, Chovanec M, Dvořánková B, Szabo P, Čada Z, Smetana K Jr (2010) Epithelial–stromal interaction in squamous cell epithelium—derived tumors: an important new player in the control of tumor biological properties. Anticancer Res 30:455–462
Polyak K, Haviv I, Campbell IG (2008) Co-evolution of tumor cells and their microenvironment. Trends Genet 25:30–38
Quante M, Tu SP, Tomita H, Gonda T, Wang SSW, Takashi S, Baik GH, Shibata W, DiPrete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272
Schürch W, Seemayer TA, Lagacé R, Gabbiani G (1984) The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol 403:323–336
Smetana K Jr, Dvořánková B, Szabo P, Strnad H, Kolář M (2013a) Role of stromal fibroblasts in cancer originated from squamous epithelia. In: Bai X (ed) Dermal fibroblasts: histological perspectives, characterization and role in disease. Nova Sciences Publishers, New York, pp 83–94
Smetana K Jr, Dvořánková B, Lacina L (2013b) Phylogeny, regeneration, ageing and cancer: role of microenvironment and possibility of its therapeutic manipulation. Folia Biol 59:207–216
Strnad H, Lacina L, Kolář M, Čada Z, Vlček Č, Dvořánková B, Betka J, Plzák J, Chovanec M, Šáchová J, Valach J, Urbanová M, Smetana K Jr (2010) Head and neck squamous cancer fibroblasts produce growth factors influencing phenotype of normal human keratinocytes. Histochem Cell Biol 133:201–211
Sugimoto H, Mundel TM, Kieran MW, Kalluri R (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Therapy 5:1640–1646
Szabo P, Kolář M, Dvořánková B, Lacina L, Štork J, Vlček Č, Strnad H, Tvrdek M, Smetana K Jr (2011) Mouse 3T3 fibroblasts under the influence of fibroblasts isolated from stroma of human basal cell carcinoma acquire properties of multipotent stem cells. Biol Cell 103:233–248
Wu Y, Zhou BP (2009) Inflammation. A driving force speeds cancer metastasis. Cell Cycle 8:3267–3273
Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R (2007) Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67:10123–10128
Acknowledgments
This study was supported by the Grant Agency of the Czech Republic, projects Nos. 13-20293S and P301/12/1254, by the grant of Grant Agency of Ministry of Health of the Czech Republic (NT13488), by the Charles University projects PRVOUK-27, UNCE 204013 and by the Specific University Research (SVV). Authors are also grateful to EC projects: BIOCEV (Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University in Vestec -CZ.1.05/1.1.00/02.0109—from the European Regional Development Fund) and the ITN network GLYCOPHARM (Contract No. 317297) for support. Authors are grateful to Marie Jindráková, Radana Kavková, Helena Mišurcová and Pavlína Jungrová for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dvořánková, B., Smetana, K., Říhová, B. et al. Cancer-associated fibroblasts are not formed from cancer cells by epithelial-to-mesenchymal transition in nu/nu mice. Histochem Cell Biol 143, 463–469 (2015). https://doi.org/10.1007/s00418-014-1293-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1293-z