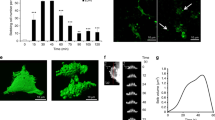
Abstract
During melanoma tumour growth, cancerous cells are exposed to the immediate surrounding the micro- and macro environment, which is largely modified through the degradation of the extracellular matrix by fibroblast-derived metalloproteinases. Among the degradation products, (VGVAPG)3, an elastin peptide is known to stimulate the proliferation of both fibroblasts and cancerous cells by binding to the elastin-binding receptor and activating the MEK/ERK signal transduction pathway. As this process strongly modifies mRNA synthesis, we investigated its effect on the relative three-dimensional organisation of the major partners of the mRNA splicing machinery: promyelocytic nuclear bodies (PML-NBs ) and splicing component 35 speckles (SC35) of normal fibroblasts and melanoma SK-MEL-28 cells. SC35 and PML-NBs proteins were immunolabeled and imaged by confocal microscopy within these cells cultured with (VGVAPG)3. Three-dimensional reconstruction was performed to elucidate the organisation of PML-NBs and SC35 speckles and their spatial relationship. In G0 cells, SC35 speckles were sequestered in PML-NBs. Shortly after (VGVAPG)3 stimulation, the three-dimensional organisation of PML-NBs and SC35 speckles changed markedly. In particular, SC35 speckles gradually enlarged and adopted a heterogeneous organisation, intermingled with PML-NBs. Conversely, inhibition of the elastin-binding protein or MEK/ERK pathway induced a remarkable early sequestration of condensed SC35 speckles in PML-NBs, the hallmark of splicing inhibition. The 3D architecture of speckles/PML-NBs highlights the modulation in their spatial relationship, the multiple roles of PML-NBs in activation, inhibition and sequestration, and provides the first demonstration of the dependence of PML-NBs and SC35 speckles on the elastin peptide for these functions.







Similar content being viewed by others
References
Bernardi R, Pandolfi PP (2007) Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol 8:1006–1016
Bissell MJ, Kenny PA, Radisky DC (2005) Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol 70:343–356
Boe SO, Haave M, Jul-Larsen A, Grudic A, Bjerkvig R et al (2006) Promyelocytic leukemia nuclear bodies are predetermined processing sites for damaged DNA. J Cell Sci 119:3284–3295
Bruckner-Tuderman L, von der Mark K, Pihlajaniemi T, Unsicker K (2010) Cell interactions with the extracellular matrix. Cell Tissue Res 339:1–5
Burke B, Stewart CL (2014) Functional architecture of the cell’s nucleus in development, aging, and disease. Curr Top Dev Biol 109:1–52
Ching WC, Kashif A, Boutros PC, Penn LZ, Bazett-Jones DP (2013) Identifying gene locus associations with promyelocytic leukemia nuclear bodies using immuno-TRAP. J Cell Biol 201:325–335
Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2:292–300
Cukierman E, Bassi DE (2010) Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol 20:139–145
Dellaire G, Ching RW, Dehghani H, Ren Y, Bazett-Jones DP (2006) The number of PML nuclear bodies increases in early S phase by a fission mechanism. J Cell Sci 119:1026–1033
Devy J, Duca L, Cantarelli B, Joseph-Pietras D, Scandolera A et al (2010) Elastin-derived peptides enhance melanoma growth in vivo by upregulating the activation of Mcol-A (MMP-1) collagenase. Br J Cancer 103:1562–1570
Dimitrova DS, Berezney R (2002) The spatio-temporal organization of DNA replication sites is identical in primary, immortalized and transformed mammalian cells. J Cell Sci 115:4037–4051
Duca L, Floquet N, Alix AJ, Haye B, Debelle L (2004) Elastin as a matrikine. Crit Rev Oncol Hematol 49:235–244
Duca L, Blanchevoye C, Cantarelli B, Ghoneim C, Dedieu S et al (2007) The elastin receptor complex transduces signals through the catalytic activity of its Neu-1 subunit. J Biol Chem 282:12484–12491
Dundr M, Misteli T (2010) Biogenesis of nuclear bodies. Cold Spring Harb Perspect Biol 2:a000711
Elias E, Lalun N, Lorenzato M, Blache L, Chelidze P et al (2003) Cell-cycle-dependent three-dimensional redistribution of nuclear proteins, P 120, pKi-67, and SC35 splicing factor, in the presence of the topoisomerase I inhibitor camptothecin. Exp Cell Res 291:176–188
English JM, Cobb JH (2002) Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci 23:40–45
Eskiw CH, Dellaire G, Mymryk JS, Bazett-Jones DP (2003) Size, position and dynamic behavior of PML nuclear bodies following cell stress as a paradigm for supramolecular trafficking and assembly. J Cell Sci 116:4455–4466
Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R et al (2005) Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res 65:4134–4146
Gribbon C, Dahm R, Prescott AR, Quinlan RA (2002) Association of the nuclear matrix component NuMA with the Cajal body and nuclear speckle compartments during transitions in transcriptional activity in lens cell differentiation. Eur J Cell Biol 81:557–566
Hall LL, Smith KP, Byron M, Lawrence JB (2006) Molecular anatomy of a speckle. Anat Rec A Discov Mol Cell Evol Biol 288:664–675
Jackman J, O’Connor PM (2001) Methods for synchronizing cells at specific stages of the cell cycle. Curr Protoc Cell Biol Chapter 8:Unit 8.3
Kamoun A, Landeau JM, Godeau G, Wallach J, Duchesnay A et al (1995) Growth stimulation of human skin fibroblasts by elastin-derived peptides. Cell Adhes Commun 3:273–281
Kiesslich A, von Mikecz A, Hemmerich P (2002) Cell cycle-dependent association of PML bodies with sites of active transcription in nuclei of mammalian cells. J Struct Biol 140:167–179
Korotayev K, Chaussepied M, Ginsberg D (2008) ERK activation is regulated by E2F1 and is essential for E2F1-induced S phase entry. Cell Signal 20:1221–1226
Kumar PP, Bischof O, Purbey PK, Notani D, Urlaub H et al (2007) Functional interaction between PML and SATB1 regulates chromatin-loop architecture and transcription of the MHC class I locus. Nat Cell Biol 9:45–56
Lallemand-Breitenbach V, de The H (2010) PML nuclear bodies. Cold Spring Harb Perspect Biol 2:a000661
Lamond AI, Spector DL (2003) Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol 4:605–612
Lang M, Jegou T, Chung I, Richter K, Munch S et al (2010) Three-dimensional organization of promyelocytic leukemia nuclear bodies. J Cell Sci 123:392–400
Lin S, Coutinho-Mansfield G, Wang D, Pandit S, Fu XD (2008) The splicing factor SC35 has an active role in transcriptional elongation. Nat Struct Mol Biol 15:819–826
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z et al (1997) Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861–1872
Lorenzato M, Caudroy S, Nou JM, Dalstein V, Joseph K, Bellefqih S, Durlach A, Thil C, Dez F, Bouttens D, Clavel C, Birembaut P (2008) Contributionof DNA ploidy image cytometry to the management of ASC cervical lesions. Cancer Cytopathol 114:263–269
Mammoto A, Mammoto T, Ingber DE (2012) Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 30:61–73
Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC (2004) An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol 49:199–202
Matsuzaki K, Minami T, Tojo M, Honda Y, Saitoh N et al (2003) PML-nuclear bodies are involved in cellular serum response. Genes Cells 8:275–286
Mintz PJ, Spector DL (2000) Compartmentalization of RNA processing factors within nuclear speckles. J Struct Biol 129:241–251
Misteli T (2000) Cell biology of transcription and pre-mRNA splicing: nuclear architecture meets nuclear function. J Cell Sci 113:1841–1849
Misteli T (2009) Self-organization in the genome. Proc Natl Acad Sci USA 106:6885–6886
Mochizuki S, Brassart B, Hinek A (2002) Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem 277:44854–44863
Rusciani A, Duca L, Sartelet H, Chatron-Colliet A, Bobichon H et al (2010) Elastin peptides signaling relies on neuraminidase-1-dependent lactosylceramide generation. PLoS One 5:e14010
Sacco-Bubulya P, Spector DL (2002) Disassembly of interchromatin granule clusters alters the coordination of transcription and pre-mRNA splicing. J Cell Biol 156:425–443
Salomoni P, Pandolfi PP (2002) The role of PML in tumor suppression. Cell 108:165–170
Schorl C, Sedivy JM (2007) Analysis of cell cycle phases and progression in cultured mammalian cells. Methods 41:143–150
Shiels C, Islam SA, Vatcheva R, Sasieni P, Sternberg MJ et al (2001) PML bodies associate specifically with the MHC gene cluster in interphase nuclei. J Cell Sci 114:3705–3716
Sosa BA, Kutay U, Schwartz TU (2013) Structural insights into LINC complexes. Curr Opin Struct Biol 23:285–291
Spector DL, Lamond AI (2010) Nuclear speckles. Cold Spring Harb Perspect Biol 3:a000612
Spencer VA, Xu R, Bissell MJ (2007) Extracellular matrix, nuclear and chromatin structure, and gene expression in normal tissues and malignant tumors: a work in progress. Adv Cancer Res 97:275–294
Tchelidze P, Chatron-Colliet A, Thiry M, Lalun N, Bobichon H et al (2009) Tomography of the cell nucleus using confocal microscopy and medium voltage electron microscopy. Crit Rev Oncol Hematol 69:127–143
Thiry M (1995) The interchromatin granules. Histol Histopathol 10:1035–1045
Wang J, Shiels C, Sasieni P, Wu PJ, Islam SA et al (2004) Promyelocytic leukemia nuclear bodies associate with transcriptionally active genomic regions. J Cell Biol 164:515–526
Xiao R, Sun Y, Ding JH, Lin S, Rose DW et al (2007) Splicing regulator SC35 is essential for genomic stability and cell proliferation during mammalian organogenesis. Mol Cell Biol 27:5393–5402
Xu R, Boudreau A, Bissell MJ (2009) Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev 28:167–176
Zhong S, Müller S, Ronchietti S, Freemont PS, Dejean A, Pandolfi PP (2000a) Role of SUMO-1 modified PML in nuclear body formation. Blood 95:2748–2753
Zhong S, Salomoni P, Pandolfi PP (2000b) The transcriptional role of PML and the nuclear body. Nat Cell Biol 2:E85–E90
Acknowledgments
This work received financial support from Région Chanpagne-Ardenne for PhD grant of Aurore Chatron-Colliet.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatron-Colliet, A., Lalun, N., Terryn, C. et al. The elastin peptide (VGVAPG)3 induces the 3D reorganisation of PML-NBs and SC35 speckles architecture, and accelerates proliferation of fibroblasts and melanoma cells. Histochem Cell Biol 143, 245–258 (2015). https://doi.org/10.1007/s00418-014-1274-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-014-1274-2