Abstract
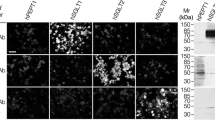
Immunohistochemical analysis was used to define the precise cell-specific localization of Glucose-6-phosphatase (Glc6Pase) and cytosolic form of the phosphoenolpyruvate carboxykinase (PEPCK-C) in the digestive system (liver, small intestine and pancreas) and the kidney. Co-expression of Glc6Pase and PEPCK-C was shown to take place in hepatocytes, in proximal tubules of the cortex kidney and at the top of the villi of the small intestine suggesting that these tissues are all able to perform complete gluconeogenesis. On the other hand, intrahepatic bile ducts, collecting tubes of the nephron and the urinary epithelium in the calices of the kidney, as well as the crypts of the small intestine, express Glc6Pase without significant levels of PEPCK-C. In such cases, the function of Glc6Pase could be related to the transepithelial transport of glucose characteristic of these tissues, rather than to the neoformation of glucose. Lastly, PEPCK-C expression in the absence of Glc6Pase was noted in both the exocrine pancreas and the endocrine islets of Langerhans. Possible roles of PEPCK-C in exocrine pancreas might be the provision of gluconeogenic intermediates for further conversion into glucose in the liver, whereas PEPCK-C would be instrumental in pyruvate cycling, which has been suggested to play a regulatory role in insulin secretion by the β-cells of the islets.







Similar content being viewed by others
Abbreviations
- Glc6Pase:
-
Glucose-6-phosphatase
- GSD 1a:
-
Glycogen storage disease type 1a
- PEPCK-C:
-
Cytosolic form of the phosphoenolpyruvate carboxykinase
- RT–PCR:
-
Reverse transcription–polymerase chain reaction
- PP:
-
Periportal
- PV:
-
Perivenous
- rL19:
-
Ribosomal protein L19
References
Arden SD, Zahn T, Steegers S, Webb S, Bergman B, O’Brien RM, Hutton JC (1999) Molecular cloning of a pancreatic islet-specific glucose-6-phosphatase catalytic subunit-related protein. Diabetes 48:531–542
Ashcroft SJ, Randle PJ (1970) Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J 119:5–15
Aspord C, Rome S, Thivolet C (2004) Early events in islets and pancreatic lymph nodes in autoimmune diabetes. J Autoimmun 23:27–35
Bodamer OA, Feillet F, Lane RE, Lee PJ, Dixon MA, Halliday D, Leonard JV (2002) Utilization of cornstarch in glycogen storage disease type Ia. Eur J Gastroenterol Hepatol 14:1251–1256
Botini FF, Suzuki-Kemmelmeier F, Nascimento EA, Ide LT, Bracht A (2005) Zonation of alanine metabolism in the bivascularly perfused rat liver. Liver Int 25:861–871
Chakravarty K, Cassuto H, Reshef L, Hanson RW (2005) Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol 40:129–154
Chamlian A, Benkoel L, Bongrand P, Gulian JM, Brisse J (1991) Quantitative histochemical study of glucose-6-phosphatase in periportal and perivenous human hepatocytes. Cell Mol Biol 37:183–190
Chou JY, Matern D, Mansfield BC, Chen YT (2002) Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med 2:121–143
Croset M, Rajas F, Zitoun C, Hurot JM, Montano S, Mithieux G (2001) Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes 50:740–746
Curthoys NP, Watford M (1995) Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15:133–159
Field JB, Epstein S, Egan T (1965) Studies in glycogen storage diseases. I. Intestinal glucose-6-phosphatase activity in patients with von gierke’s disease and their parents. J Clin Invest 44:1240–1247
Flamez D, Berger V, Kruhoffer M, Orntoft T, Pipeleers D, Schuit FC (2002) Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes 51:2018–2024
Gerich JE, Meyer C, Woerle HJ, Stumvoll M (2001) Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24:382–391
Guder WG, Ross BD (1984) Enzyme distribution along the nephron. Kidney Int 26:101–111
Guignot L, Mithieux G (1999) Mechanisms by which insulin, associated or not with glucose, may inhibit hepatic glucose production in the rat. Am J Physiol 277:E984–E989
Guzelian P, Boyer JL (1974) Glucose reabsorption from bile. Evidence for a biliohepatic circulation. J Clin Invest 53:526–535
Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH, Oudart H (2005) Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol 566:575–586
Hanson RW, Reshef L (1997) Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66:581–611
Hanson RW, Reshef L (2003) Glyceroneogenesis revisited. Biochimie 85:1199–1205
Hedeskov CJ, Capito K, Thams P (1984) Phosphoenolpyruvate carboxykinase in mouse pancreatic islets. ATP-induced changes in sensitivity to Mn2+ activation. Biochim Biophys Acta 791:37–44
Hildebrand R, Schleicher A (1986) Image analysis of the histochemical demonstration of glucose-6-phosphatase activity in rat liver. Histochemistry 86:181–190
Hume R, Bell JE, Hallas A, Burchell A (1994) Immunohistochemical localization of glucose-6-phosphatase in developing human kidney. Histochemistry 101:413–417
Iynedjian PB, Peters G (1974) Phosphoenolpyruvate carboxykinase and gluconeogenesis in renal cortex of starved rats. Am J Physiol 226:1281–1285
Iynedjian PB, Jacot MM (1980) Glucocorticoid-dependent induction of mRNA coding for phosphoenolpyruvate carboxykinase (GTP) in rat kidney. Its inhibition by cycloheximide. Eur J Biochem 111:89–98
Jitrapakdee S, Vidal-Puig A, Wallace JC (2006) Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell Mol Life Sci
Jonges GN, Van Noorden CJ, Gossrau R (1990) Quantitative histochemical analysis of glucose-6-phosphatase activity in rat liver using an optimized cerium-diaminobenzidine method. J Histochem Cytochem 38:1413–1419
Jungermann K, Heilbronn R, Katz N, Sasse D (1982) The glucose/glucose-6-phosphate cycle in the periportal and perivenous zone of rat liver. Eur J Biochem 123:429–436
Jungermann K (1992) Role of intralobular compartmentation in hepatic metabolism. Diabete Metab 18:81–86
Kamm DE, Fuisz RE, Goodman AD, Cahill GF Jr (1967) Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest 46:1172–1177
Lei KJ, Chen H, Pan CJ, Ward JM, Mosinger B Jr, Lee EJ, Westphal H, Mansfield BC, Chou JY (1996) Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet 13:203–209
Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD (2002) 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci USA 99:2708–2713
MacDonald MJ, Chang CM (1985) Do pancreatic islets contain significant amounts of phosphoenolpyruvate carboxykinase or ferroactivator activity? Diabetes 34:246–250
MacDonald MJ, McKenzie DI, Walker TM, Kaysen JH (1992) Lack of glyconeogenesis in pancreatic islets: expression of gluconeogenic enzyme genes in islets. Horm Metab Res 24:158–160
MacDonald MJ (1995) Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem 270:20051–20058
MacDonald MJ (2002) Differences between mouse and rat pancreatic islets: succinate responsiveness, malic enzyme, and anaplerosis. Am J Physiol Endocrinol Metab 283:E302–E310
MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA (2005) Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 288:E1–E15
Manz F, Bickel H, Brodehl J, Feist D, Gellissen K, Gescholl-Bauer B, Gilli G, Harms E, Helwig H, Nutzenadel W, et al (1987) Fanconi-Bickel syndrome. Pediatr Nephrol 1:509–518
Martin CC, Bischof LJ, Bergman B, Hornbuckle LA, Hilliker C, Frigeri C, Wahl D, Svitek CA, Wong R, Goldman JK, Oeser JK, Lepretre F, Froguel P, O’Brien RM, Hutton JC (2001) Cloning and characterization of the human and rat islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) genes. J Biol Chem 276:25197–25207
Masyuk AI, Masyuk TV, Tietz PS, Splinter PL, LaRusso NF (2002) Intrahepatic bile ducts transport water in response to absorbed glucose. Am J Physiol Cell Physiol 283:C785–C791
Minassian C, Zitoun C, Mithieux G (1996) Differential time course of liver and kidney glucose-6 phosphatase activity during long-term fasting in rat correlates with differential time course of messenger RNA level. Mol Cell Biochem 155:37–41
Mithieux G, Vidal H, Zitoun C, Bruni N, Daniele N, Minassian C (1996) Glucose-6-phosphatase mRNA and activity are increased to the same extent in kidney and liver of diabetic rats. Diabetes 45:891–896
Mithieux G, Bady I, Gautier A, Croset M, Rajas F, Zitoun C (2004a) Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 286:370–375
Mithieux G, Rajas F, Gautier-Stein A (2004b) A novel role for glucose 6-phosphatase in the small intestine in the control of glucose homeostasis. J Biol Chem 279:44231–44234
Mithieux G, Misery P, Magnan C, Pillot B, Gautier-Stein A, Bernard C, Rajas F, Zitoun C (2005) Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab 2:321–329
Mithieux G, Gautier-Stein A, Rajas F, Zitoun C (2006) Contribution of intestine and kidney to glucose fluxes in different nutritional states in rat. Comp Biochem Physiol B Biochem Mol Biol 143:195–200
Portha B, Giroix MH, Serradas P, Gangnerau MN, Movassat J, Rajas F, Bailbe D, Plachot C, Mithieux G, Marie JC (2001) beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes 50:S89–S93
Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G (1999) The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 117:132–139
Rajas F, Croset M, Zitoun C, Montano S, Mithieux G (2000) Induction of PEPCK gene expression in insulinopenia in rat small intestine. Diabetes 49:1165–1168
Santer R, Steinmann B, Schaub J (2002) Fanconi-Bickel syndrome-a congenital defect of facilitative glucose transport. Curr Mol Med 2:213–227
Santer R, Hillebrand G, Steinmann B, Schaub J (2003) Intestinal glucose transport: evidence for a membrane traffic-based pathway in humans. Gastroenterology 124:34–39
Short MK, Clouthier DE, Schaefer IM, Hammer RE, Magnuson MA, Beale EG (1992) Tissue-specific, developmental, hormonal, and dietary regulation of rat phosphoenolpyruvate carboxykinase-human growth hormone fusion genes in transgenic mice. Mol Cell Biol 12:1007–1020
Simpson NE, Khokhlova N, Oca-Cossio JA, Constantinidis I (2006) Insights into the role of anaplerosis in insulin secretion: a (13) C NMR study. Diabetologia 49:1338–1348
Stumpel F, Burcelin R, Jungermann K, Thorens B (2001) Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci USA 98:11330–11335
Waddell ID, Burchell A (1988) The microsomal glucose-6-phosphatase enzyme of pancreatic islets. Biochem J 255:471–476
Yanez AJ, Nualart F, Droppelmann C, Bertinat R, Brito M, Concha II, Slebe JC (2003) Broad expression of fructose-1, 6-bisphosphatase and phosphoenolpyruvate carboxykinase provide evidence for gluconeogenesis in human tissues other than liver and kidney. J Cell Physiol 197:189–197
Zimmer DB, Magnuson MA (1990) Immunohistochemical localization of phosphoenolpyruvate carboxykinase in adult and developing mouse tissues. J Histochem Cytochem 38:171–178
Acknowledgments
The authors thank the INSERM and INRA for funding their work, and the CNRS (G. M., F. R., A. S.) for funding their position. The sections were realized by N. Gadot (ANIPATH, Faculty of Medecine Laennec, Lyon). We thank P. Chevallier for her advisements in immunohistochemistry and Dr. AM. Madec and Prof. J. Trouillas for histology. The assistance of C. Vanbelle (INSERM, IFR62) for image analyses is gratefully acknowledged. We would also like to thank Dr. D. Granner who provided us sheep anti-PEPCK antibodies used for comparative purposes.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00418-007-0270-1
Rights and permissions
About this article
Cite this article
Rajas, F., Jourdan-Pineau, H., Stefanutti, A. et al. Immunocytochemical localization of glucose 6-phosphatase and cytosolic phosphoenolpyruvate carboxykinase in gluconeogenic tissues reveals unsuspected metabolic zonation. Histochem Cell Biol 127, 555–565 (2007). https://doi.org/10.1007/s00418-006-0263-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-006-0263-5