Abstract.
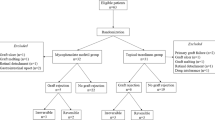
Background: With the use of systemic cyclosporin A (CsA), graft prognosis after high-risk penetrating keratoplasty has improved considerably. However, the application of CsA is limited owing to a variety of severe side effects. In this prospectively randomized study mycophenolate mofetil (MMF), a safe and efficient immunosuppressive medication after renal transplantation, was compared with CsA after high-risk penetrating keratoplasty. Methods: Twenty-nine high-risk keratoplasty patients were treated with MMF 2× 1 g daily; another 27 patients received CsA, aiming at blood trough levels of 120–150 ng/ml. Systemic immunosuppression was scheduled for 6 months. In both groups oral corticosteroids (fluocortolone 1 mg/kg) were administered for 3 weeks postoperatively. Results: During the first year after operation, no graft failure was recorded. Two years postoperatively 92%/82% and 3 years postoperatively 74%/69% of grafts were clear in the MMF and CsA group, respectively (Kaplan Meier P=0.33, log-rank test). In total, two graft failures were recorded in the MMF group and four in the CsA group. Three years postoperatively 53% of the grafts were rejection-free in the MMF group and 73% in the CsA group (Kaplan Meier P=0.46, log-rank test). Eight endothelial immune reactions were observed in the MMF group (three under systemic immunosuppression/five thereafter; six mild/two severe) and five in the CsA group (three under systemic immunosuppression/two thereafter; three mild/two severe). Side effects occurred in six patients under MMF and 11 under CsA. Conclusions: Concerning efficacy, no statistically significant difference between systemic MMF and systemic CsA administered for 6 months after high-risk penetrating keratoplasty could be shown. Systemic MMF was proven to be at least as safe as CsA. The main mechanism in improving graft survival is a shift from severe to milder endothelial immune reactions, as already demonstrated for CsA. Thus, MMF may become an alternative to CsA for immunosuppression after penetrating high-risk keratoplasty. About 2 years postoperatively, pharmacologically induced relative immunological tolerance slowly decreases. Therefore, long-term administration of systemic MMF should be evaluated in further studies.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Reinhard, T., Reis, A., Böhringer, D. et al. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years' results of a randomized prospective clinical trial. Graefe's Arch Clin Exp Ophthalmol 239, 367–372 (2001). https://doi.org/10.1007/s004170100285
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004170100285