Abstract
Purpose
To create a model for prediction of postoperative visual acuity (VA) after vitrectomy for macular hole (MH) treatment using preoperative optical coherence tomography (OCT) images, using deep learning (DL)–based artificial intelligence.
Methods
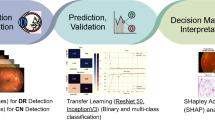
This was a retrospective single-center study. We evaluated 259 eyes that underwent vitrectomy for MHs. We divided the eyes into four groups, based on their 6-month postoperative Snellen VA values: (A) ≥ 20/20; (B) 20/25–20/32; (C) 20/32–20/63; and (D) ≤ 20/100. Training data were randomly selected, comprising 20 eyes in each group. Test data were also randomly selected, comprising 52 total eyes in the same proportions as those of each group in the total database. Preoperative OCT images with corresponding postoperative VA values were used to train the original DL network. The final prediction of postoperative VA was subjected to regression analysis based on inferences made with DL network output. We created a model for predicting postoperative VA from preoperative VA, MH size, and age using multivariate linear regression. Precision values were determined, and correlation coefficients between predicted and actual postoperative VA values were calculated in two models.
Results
The DL and multivariate models had precision values of 46% and 40%, respectively. The predicted postoperative VA values on the basis of DL and on preoperative VA and MH size were correlated with actual postoperative VA at 6 months postoperatively (P < .0001 and P < .0001, r = .62 and r = .55, respectively).
Conclusion
Postoperative VA after MH treatment could be predicted via DL using preoperative OCT images with greater accuracy than multivariate linear regression using preoperative VA, MH size, and age.






Similar content being viewed by others
References
Gass JD (1988) Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 106:629–639. https://doi.org/10.1001/archopht.1988.01060130683026
Kelly NE, Wendel RT (1991) Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 109:654–659. https://doi.org/10.1001/archopht.1991.01080050068031
Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J (1999) Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology 106:1392–1398. https://doi.org/10.1016/s0161-6420(99)00730-7
Brooks HL (2000) Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 107:1939–1948. https://doi.org/10.1016/s0161-6420(00)00331-6
Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A (2012) Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology 119:1438–1446. https://doi.org/10.1016/j.ophtha.2012.01.023
Baba T, Kakisu M, Nizawa T, Oshitari T, Yamamoto S (2017) Superficial foveal avascular zone determined by optical coherence tomography angiography before and after macular hole surgery. Retina 37:444–450. https://doi.org/10.1097/IAE.0000000000001205
Amram AL, Mandviwala MM, Ou WC, Wykoff CC, Shah AR (2018) Predictors of visual acuity outcomes following vitrectomy for idiopathic macular hole. Ophthalmic Surg Lasers Imaging Retina 49:566–570. https://doi.org/10.3928/23258160-20180803-03
Kim SH, Kim HK, Yang JY, Lee SC, Kim SS (2018) Visual recovery after macular hole surgery and related prognostic factors. Korean J Ophthalmol 32:140–146. https://doi.org/10.3341/kjo.2017.0085
Kumagai K, Ogino N, Demizu S, Atsumi K, Kurihara H, Iwaki M, Ishigooka H, Tachi N (2000) Factors related to initial success in macular hole surgery. Nippon Ganka Gakkai Zasshi 104:792–796
Kokame GT, de Bustros S (1995) Visual acuity as a prognostic indicator in stage I macular holes. Am J Ophthalmol 120:112–114. https://doi.org/10.1016/s0002-9394(14)73768-7
Ullrich S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A (2002) Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol 86:390–393. https://doi.org/10.1136/bjo.86.4.390
Scott RA, Ezra E, West JF, Gregor ZJ (2000) Visual and anatomical results of surgery for long standing macular holes. Br J Ophthalmol 84:150–153. https://doi.org/10.1136/bjo.84.2.150
Jaycock PD, Bunce C, Xing W, Thomas D, Poon W, Gazzard G, Williamson TH, Laidlaw DA (2005) Outcomes of macular hole surgery: implications for surgical management and clinical governance. Eye (Lond) 19:879–884. https://doi.org/10.1038/sj.eye.6701679
Gupta B, Laidlaw DA, Williamson TH, Shah SP, Wong R, Wren S (2009) Predicting visual success in macular hole surgery. Br J Ophthalmol 93:1488–1491. https://doi.org/10.1136/bjo.2008.153189
Larsson J, Holm K, Lovestam-Adrian M (2006) The presence of an operculum verified by optical coherence tomography and other prognostic factors in macular hole surgery. Acta Ophthalmol Scand 84:301–304. https://doi.org/10.1111/j.1600-0420.2006.00672.x
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444. https://doi.org/10.1038/nature14539
Lee CS, Tyring AJ, Deruyter NP, Wu Y, Rokem A, Lee AY (2017) Deep-learning based, automated segmentation of macular edema in optical coherence tomography. Biomed Opt Express 8:3440–3448. https://doi.org/10.1364/BOE.8.003440
Lu D, Heisler M, Lee S, Ding GW, Navajas E, Sarunic MV, Beg MF (2019) Deep-learning based multiclass retinal fluid segmentation and detection in optical coherence tomography images using a fully convolutional neural network. Med Image Anal 54:100–110. https://doi.org/10.1016/j.media.2019.02.011
Alsaih K, Yusoff MZ, Tang TB, Faye I, Meriaudeau F (2020) Deep learning architectures analysis for age-related macular degeneration segmentation on optical coherence tomography scans. Comput Methods Programs Biomed 195:105566. https://doi.org/10.1016/j.cmpb.2020.105566
Asaoka R, Murata H, Hirasawa K, Fujino Y, Matsuura M, Miki A, Kanamoto T, Ikeda Y, Mori K, Iwase A, Shoji N, Inoue K, Yamagami J, Araie M (2019) Using deep learning and transfer learning to accurately diagnose early-onset glaucoma from macular optical coherence tomography images. Am J Ophthalmol 198:136–145. https://doi.org/10.1016/j.ajo.2018.10.007
Russakoff DB, Mannil SS, Oakley JD, Ran AR, Cheung CY, Dasari S, Riyazzuddin M, Nagaraj S, Rao HL, Chang D, Chang RT (2020) A 3D deep learning system for detecting referable glaucoma using full OCT macular cube scans. Transl Vis Sci Technol 9:12. https://doi.org/10.1167/tvst.9.2.12
Nagasato D, Tabuchi H, Masumoto H, Enno H, Ishitobi N, Kameoka M, Niki M, Mitamura Y (2019) Automated detection of a nonperfusion area caused by retinal vein occlusion in optical coherence tomography angiography images using deep learning. PLoS ONE 14:e0223965. https://doi.org/10.1371/journal.pone.0223965
Sonobe T, Tabuchi H, Ohsugi H, Masumoto H, Ishitobi N, Morita S, Enno H, Nagasato D (2019) Comparison between support vector machine and deep learning, machine-learning technologies for detecting epiretinal membrane using 3D-OCT. Int Ophthalmol 39:1871–1877. https://doi.org/10.1007/s10792-018-1016-x
De Fauw J, Ledsam JR, Romera-Paredes B, Nikolov S, Tomasev N, Blackwell S, Askham H, Glorot X, O’Donoghue B, Visentin D, van den Driessche G, Lakshminarayanan B, Meyer C, Mackinder F, Bouton S, Ayoub K, Chopra R, King D, Karthikesalingam A, Hughes CO, Raine R, Hughes J, Sim DA, Egan C, Tufail A, Montgomery H, Hassabis D, Rees G, Back T, Khaw PT, Suleyman M, Cornebise J, Keane PA, Ronneberger O (2018) Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med 24:1342–1350. https://doi.org/10.1038/s41591-018-0107-6
Kawczynski MG, Bengtsson T, Dai J, Hopkins JJ, Gao SS, Willis JR (2020) Development of deep learning models to predict best-corrected visual acuity from optical coherence tomography. Transl Vis Sci Technol 9:51. https://doi.org/10.1167/tvst.9.2.51
Kingma D BJ Adam: (2014) A method for stochastic optimization. arXiv preprint arXiv:14126980
Lovie-Kitchin JE, Whittaker SG (1999) Prescribing near magnification for low vision patients. Clin Exp Optom 82:214–224. https://doi.org/10.1111/j.1444-0938.1999.tb06651.x
Szegedy C, Vanhoucke V, Ioffe S, Shlens J, Wojna Z (2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 2016, pp. 2818–2826, https://doi.org/10.1109/CVPR.2016.308.) Rethinking the Inception Architecture for Computer Vision,
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D (2019:618e626) Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vis
Chawla NV, Japkowicz N, Kotcz A (2004) Editorial ACM SIGKDD Explorations Newsletter 6:1–6. https://doi.org/10.1145/1007730.1007733
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Wendel RT, Patel AC, Kelly NE, Salzano TC, Wells JW, Novack GD (1993) Vitreous surgery for macular holes. Ophthalmology 100:1671–1676. https://doi.org/10.1016/s0161-6420(93)31419-3
Acknowledgements
The analysis using AI based on DL was outsourced to and conducted in collaboration with Tiwaki Co., Ltd. We thank Yoshiki Ohashi, Taro Watasue, Tomohiro Nakagawa, and Xiang Ruan for contributions to our deep learning analysis. We thank Ryan Chastain-Gross, Ph.D., from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript. The Clinical Research and Advanced Center at Shiga University of Medical Science assisted with statistical analysis.
Funding
Funding for this study was provided by Shiga University of Medical Sciences. The Department of Ophthalmology (Shiga University of Medical Science) provides financial support to Tiwaki Co., Ltd. The funding body had no further input into the collection, analysis, and interpretation of the data, or manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board (IRB)/Ethics Committee Shiga University of Medical Science (Otsu, Japan).
Consent to participate
For this type of study, formal consent was not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obata, S., Ichiyama, Y., Kakinoki, M. et al. Prediction of postoperative visual acuity after vitrectomy for macular hole using deep learning–based artificial intelligence. Graefes Arch Clin Exp Ophthalmol 260, 1113–1123 (2022). https://doi.org/10.1007/s00417-021-05427-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05427-2