Abstract
Purpose
To assess the relationship between the axial length and post-LASIK regression in myopic patients.
Methods
This is a retrospective case series study conducted at a private eye centre, Ismailia, Egypt. The clinical records of the patients, who experienced LASIK to correct myopia from January 2016 to January 2018, were analysed for myopic regression. The patients were operated on, examined, and followed-up 1 year by one surgeon (AAG).
Results
This study included 1219 patients (2316 eyes) with myopia. Mean ± SD of pre-operative spherical equivalent (SE) was − 4.3 ± 2.1D, range (− 0.50 to − 10.0D). Mean ± SD age of the patients was 26.4 ± 6.8 years, range (21 to 50 years). Male to female ratio was 30.5 to 69.5%. The cumulative incidence rate of myopic regression according to the medical records of the patients was 25.12% (582 eyes out of total 2316 eyes) along the 2 years of this study (12.6% per year). Of the total patients, 14.94% had pre-operative high myopia, 35.84% had pre-operative moderate myopia, and 49.2% had pre-operative low myopia. Of the patients with myopic regression, 52.6% had pre-operative high myopia, 34% had pre-operative moderate myopia, and 13.4% had pre-operative low myopia. The mean ± SD of the axial length of the patients with myopic regression was 26.6 ± 0.44 mm, range (26.0 to 27.86 mm), while the mean ± SD of the axial length of other patients with stable refraction was 24.38 ± 0.73 mm, range (22.9 to 25.9 mm) (t test statistic = 69.3; P value < 0.001).
Conclusions
Pre-operative high axial length increases the risk of myopic regression after LASIK.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-laser in situ keratomileusis myopic regression can be defined as gradual, incomplete, or complete loss of primary correction that limits the efficiency, predictability, and long-term stability of laser in situ keratomileusis (LASIK) [1].
Although LASIK techniques and surgeon experience have improved over the past 20 years resulting in better outcomes, myopic regression after LASIK is inevitable, and the exact mechanism of it is unclear and under-explored [2]. Previous studies had recorded some regression-related factors before and after LASIK and suggested a forward shift of the cornea as an explanation of myopic regression [3,4,5]. Patel et al. [6] recorded a myopic regression rate of 16% along the 3-month follow-up period of their study. Hersh et al. [7] recorded a 10.5% incidence of myopic regression in their study (3-year follow-up period). They stated that high primary corrections, astigmatism, and old age were significant risk factors for it. Liu et al. [8] recorded a rate of 21% myopic regression along 5 years of their study. Randleman et al. [9] recorded a 6.3% enhancement rate along 1-year follow-up period and stated that patients with high myopic errors were more expected to have enhancement. In long-term studies, Alio et al. [10] recorded retreatment rates ranging from 20 to 27% in their study with 10 years follow-up.
Another factor that might influence the post-LASIK myopic regression is the axial length of the eye. So, this study aimed to assess the relationship between refractive regression and the axial length in myopic patients.
Patients and methods
This is a retrospective and case series study that included patients who experienced LASIK to correct myopia from January 2016 to January 2018, at a private eye centre, Ismailia, Egypt.
Inclusion criteria were age over 21 years, intraocular pressure (IOP) less than 21 mmHg, central corneal thickness (CCT) > 500 μm at the thinnest point and the calculated residual stromal bed after treatment > 60% of the total corneal thickness, a regular corneal topography pattern (Sirius, CSO, Florence, Italy), and no history of diabetes mellitus, autoimmune diseases, or earlier ocular surgeries. Patients with insufficient follow-up were excluded from the study.
Pre-operatively, patients experienced standard eye examinations, including slit-lamp examination, indirect fundoscopy, and refraction (cycloplegic and manifest and presented as spherical equivalent − 0.5 to − 10.00D), intraocular pressure (IOP) by applanation tonometry and ocular response analyser (ORA), and axial length (Lenstar, HAAG-STREIT, USA).
Laser in situ keratomileusis was performed using 500 kHz Amaris excimer laser (Schwind eye-tech-solutions, Kleinostheim, Germany). Corneal flaps with superior hinge were cut with Moria M2 microkeratome (Moria, Antony, France). The optical zones ranged from 5.8 to 7.0 mm in diameter. The patients had routine procedures. All surgeries targeted distance vision.
Post-operatively, the patients with stable refraction were examined at the 1st day; 1st week; 1st, 3rd, and 6th months, and 1 year after the surgery. Patients with myopic regression were examined monthly until enhancement. The patients were assessed by complete ocular examinations. The main outcome measures were refraction (cycloplegic and manifest) and corneal topography. Patients with topographic signs suggesting corneal ectasia, under correction, corneal haze, or other complications were excluded from the study. The patients with myopic regression were only included in this study. Patients were encouraged to return after surgery for an examination if their vision declined. Enhancement surgery was offered when needed (patient dissatisfaction with the visual result). Enhancement was done by re-lifting the flap and treating the stromal bed for all patients with myopic regression (no dropout). The patients were operated on, examined, and followed-up by one surgeon (AAG).
Data collection
The medical records of the patients with refractive regressions were reviewed. The demographic and pre-operative data were collected (age, sex, pre-operative refraction, axial length, keratometric reading, and corneal topography). In this study, refractive myopic regression will be considered as a myopic shift of ≥ 0.5D in cycloplegic refraction after LASIK full correction.
The tenets of the Helsinki Declaration were followed in this study. It was reviewed and agreed by the Faculty of Medicine, Suez Canal University research ethics committee. Informed consent was not necessary for the analysis of the medical records due to the retrospective design of the study and the large sample size.
Statistical analysis
All data manipulation and analysis were performed by the Statistical Package for the Social Sciences (SPSS) version 25 (IBM Corporation, NY, USA). Parameters of the study groups were presented as frequencies and percentages or mean values and standard deviations. The student’s t test was used to compare the differences between means in the groups. Differences between frequencies in the groups were compared by the Chi-square test or Fisher’s exact test (if > 20% of expected values were less than 5). To compare the difference in the mean measurements between the sub-groups of myopia, one-way ANOVA was performed. Shapiro-Wilk’s test was used to test for data normality. Graphs were performed with GraphPad Prism (version 5.00 for Windows, GraphPad Software, La Jolla, CA, USA). A p value < 0.05 was considered statistically significant.
Results
This study included 1219 patients (2316 eyes) with myopia. Mean ± SD of pre-operative spherical equivalent (SE) was − 4.3 ± 2.1D, range (− 0.50 to − 10.00D). Mean ± SD age of the patients was 26.4 ± 6.8 years, range (21 to 50 years). Male to female ratio was 30.5 to 69.5%. Mean ± SD of the K min was 43.3 ± 1.7D, range (38.5 to 48.9D), and of the K max was 44.8 ± 1.2 D, range (39.9 to 49.6D). The cumulative incidence rate of myopic regression according to the medical records of the patients was 25.12% (582 eyes out of total 2316 eyes) along the 2 years of this study (12.6% per year). The characteristics of all patients are presented in Table 1.
The total myopic eyes were classified according to the pre-operative refraction into three sub-groups: low myopia > − 3.0D, moderate myopia − 3.0 to > − 6.0D, and high myopia ≤ − 6.0D. In total, 49.2% had pre-operative low myopia with mean ± SD of − 2.3 ± 0.7, 35.84% had pre-operative moderate myopia with mean ± SD of − 4.9 ± 1.2, and 14.94% had pre-operative high myopia with mean ± SD of − 8.4 ± 1.8. The characteristics of each group are presented in Table 2.
Eyes with post-LASIK myopic regression were classified according to their pre-operative refraction into three sub-groups:
-
13.4% had pre-operative low myopia with mean ± SD − 2.75 ± 0.1D.
-
34% had pre-operative moderate myopia with mean ± SD − 4.43 ± 0.73D.
-
52.6% of the patients had pre-operative high myopia with mean ± SD − 7.91 ± 1.45D.
The characteristics of those patients are presented in Table 3.
The mean ± SD of the axial length of the patients with myopic regression was 26.6 ± 0.44 mm, range (26.0 to 27.86 mm), while the mean ± SD of the axial length of other patients with stable refraction was 24.38 ± 0.73 mm, range (22.9 to 25.9 mm) (t test statistic = 69.3; P value < 0.001). The characteristics of the eyes with post-LASIK stable refractions and the eyes with myopic regression are presented in Table 4 and Fig. 1. The mean ± SD of the time between initial correction and regression was 3.0 ± 1.0 months; Fig. 2 shows the survival curve of the total eyes. About 3% of all studied eyes developed myopic regression at the 1st month, compared to 5.8%, 1.6%, 0.9%, and 0.1% at 3rd, 4th, 5th, and 6th month, respectively. Figure 3 shows the survival curve of post-LASIK myopic regression according to the pre-operative myopic sub-groups. There were significantly unequal survival distributions for the different levels of pre-operative myopic sub-groups (Log Rank test = 590.3; P value < 0.001). Compared to eyes with pre-operative low myopia, eyes with pre-operative high and moderate myopia had significantly less mean survival time (9.99 and 3.99, versus 11.38 months). Further, eyes with pre-operative high myopia developed myopic regression at earlier months of the follow-up period, compared to eyes with moderate and low myopia (1st versus 3rd month).
The higher degrees of myopia were predictors of the need for retreatment. In total, 88.4% of the patients with pre-operative high myopia and axial length ≥ 26 mm had myopic regression, 23.8% of the patients with pre-operative moderate myopia and axial length ≥ 26 mm have myopic regression and 6.8% of the patients with pre-operative low myopia, and axial length ≥ 26 mm have myopic regression (Table 5 and Fig. 4). Figure 5 describes the scatter plot of axial length versus myopic regression. It shows that the amount of myopic regression was significantly and positively correlated with the pre-operative axial length (Pearson’s correlation coefficient = 0.597; P value < 0.001).
In the classification of the eyes with post-LASIK myopic regression regarding the degree of regression, in 77 eyes (13.2%), the myopic regression was < 1.0D, in 246 eyes (42.2%), the regression was 1.0 to 2.0D, and in 260 eyes (44.6%), the regression was > 2.0 D (Fig. 6).
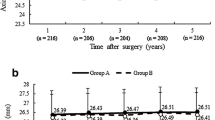
The differences between mean K values at the 1st month, 6th month, and 12th month post-operative in both eyes with stable refraction and eyes with myopic regression were non-significant (P1 = 0.154 and P2 = 0.970, respectively) (Table 6). The differences between the pre-operative axial length and along the 12-month follow-up time of both eyes with stable refraction and eyes with myopic regression are presented in Table 7. There was a statistically significant increase in the axial length (p < 0.001*) along follow-up time in eyes with myopic regression compared to eyes with stable refraction (P = 0.580). Mean ± SD of spherical equivalent (SE) along the 12-month follow-up time of both eyes with stable refraction and after LASIK enhancement in eyes with myopic regression are presented in Table 8. At the end of the follow-up time, eyes with stable refraction had a mean ± SD of + 0.21 ± 0.1D, and eyes with myopic regressions had a mean ± SD of − 0.99 ± 0.39D. Figure 7 shows the spherical equivalent (SE) pre-operatively and at 1st-day, 1st-week, 1st-month, 3rd-month, 6th-month, and the 12th-month follow-up time after LASIK in the three myopic sub-groups with stable refraction. Spherical equivalent (SE) reduction was statistically significant from the 1st day post-LASIK (P < 0.001*), with no myopic regression during the remaining follow-up time.
Discussion
High pre-operative sphere, high astigmatism, corneal steepness, and older age have been well recognized to be associated with retreatment significantly [10,11,12]. Post-LASIK compensatory epithelial hyperplasia (CEH) is still a debate between researchers, and some report post-LASIK increase in CEH and causes of myopic regression [13,14,15]; however, others [12, 16] did not report any correlation between CEH and regression.
As far as it is known, this retrospective study is the first to analyse the relationship between the refractive regression and the axial length in myopic patients. It reports that pre-operative high axial length ≥ 26 mm increases the risk of having myopic regression after LASIK. In this study, 25.13% of the patients have an axial length ≥ 26 mm, and all these patients have a myopic regression with varying degrees according to the pre-operative primary treatment. The term myopic regression suggests the loss of effect from the LASIK ablation delivered to match a certain level of myopic refraction at the initial treatment. It suggests a reduction as opposed to the term “progression” which better defines what has happened to these patients. The term LASIK regression would suggest reduction in effect from the laser ablative procedure and not progressive myopia or increase in axial length as deduced by this paper.
Some researchers [17] stated that by the age of 13 years, the axial length of the eye reaches the adult length. So, the eye could not elongate later on. On the other hand, Gudmundsdottir and associates [18] supported that the axial length of the eye changes in adults. They recorded that the mean axial length 23.6 ± 1.1 mm decreased to 23.2 ± 1.4 mm along with the 9 years of the study in 50-year-old participants. Fotedar and associates [19] recorded that with age, axial length decreased from a mean of 23.6 to 23.2 mm in the patients aged 85 years along with the 10-year study.
On the other hand, McBrien and Adams [20] investigated the biometric and refractive changes with myopia in adults (average of − 3.74D). They recorded that 48% of the study sample had an increase in the myopia of 0.37D or more during the 2-year time of the study, and this result was because of the elongation of the vitreous body. Fledelius and Goldschmidt [21] recorded a statistically significant increase in the mean of the axial length from 26.7 ± 1.3 mm at 26 years old to 27.5 ± 2.1 mm at 54 years old. Some researchers [22, 23] also proposed that, when myopia increases with age, we have to think about the potential increase of axial length in adding to suspect the development of nuclear cataract. These show that in adults, increased myopia with age is because of an increased depth of the vitreous cavity which causes an elongation of the axial length of the eye regardless of the degree of myopia.
Present results strongly suggest that patients with high axial length (≥ 26 mm) might be increasingly vulnerable to mechanical factors, for example, changing in IOP that can cause stretching of the sclera and increasing the length of the vitreous cavity because of a thin sclera which is considered causing the refractive regression. Also, the relationship between axial length and corneal biomechanics changes after the LASIK was not fully understood and needs further investigations. Wong YZ and Lam AK [24] concluded that patients with high axial length exhibited lower corneal hysteresis than emmetropes. Bueno-Gimeno et al. [25] also recorded that lower levels of corneal hysteresis were associated with longer axial length, and corneal biomechanical properties appeared to be compromised in myopia from an early age, mainly in high myopia. However, some eyes without a posterior staphyloma at the time of the primary treatment may develop it later on overtime.
The present study has some limitations: it is a retrospective study; only successive patients who completed the follow-up period were included (considering the patients who did not complete the follow-up regimen as satisfied patients with their post-LASIK refractive outcome).
In summary, our results support that pre-operative high axial length significantly increases the risk of having myopic regression after LASIK. It should be one of the pre-operative assessment measures for LASIK patients because it could give us an essential clue of post-operative stability and efficacy, not only in high myopic patients but also in low myopic patients as some of them have long axial length. These patients should be closely monitored and followed up regularly.
References
Yan MK, Chang JS, Chan TC (2018) Refractive regression after laser in situ keratomileusis. Clin Exp Ophthalmol 46(8):934–944
Lim SA, Park Y, Cheong YJ, Na KS, Joo CK (2016) Factors affecting long-term myopic regression after laser in situ keratomileusis and laser-assisted subepithelial keratectomy for moderate myopia. Korean J Ophthalmol 30(2):92–100
Miyata K, Tokunaga T, Nakahara M, Ohtani S, Nejima R, Kiuchi T, Kaji Y, Oshika T (2004) Residual bed thickness and corneal forward shift after laser in situ keratomileusis. J Cataract Refact Surg 30(5):1067–1072
Lin MY, Chang DC, Hsu WM, Wang IJ (2012) Cox proportional hazards model of myopic regression for laser in situ keratomileusis flap creation with a femtosecond laser and with a mechanical microkeratome. J Cataract Refract Surg 38(6):992–999
Ambrósio R Jr (2019) Post-LASIK ectasia: twenty years of a conundrum. Semin Ophthalmol 34(2):66–68
Patel NP, Clinch TE, Weis JR, Ahn C, Lundergan MK, Heidenreich K (2000) Comparison of visual results in initial and retreatment laser in situ keratomileusis procedures for myopia and astigmatism. Am J Ophthalmol 130(1):1–11
Hersh PS, Fry KL, Bishop DS (2003) Incidence and associations of retreatment after LASIK. Ophthalmology 110(4):748–754
Liu M, Gao H, Shi W (2019) Factors affecting myopic regression after laser in situ keratomileusis and laser-assisted subepithelial keratectomy for high myopia. Semin Ophthalmol 34(5):359–364
Randleman JB, White AJ Jr, Lynn MJ, Hu MH, Stulting RD (2009) Incidence, outcomes, and risk factors for retreatment after wavefront-optimized ablations with PRK and LASIK. J Refract Surg 25(3):273–276
Alio JL, Muftuoglu O, Ortiz D, Pérez-Santonja JJ, Artola A, Ayala MJ, Garcia MJ, de Luna GC (2008) Ten-year follow-up of laser in situ keratomileusis for myopia of up to −10 diopters. Am J Ophthalmol 145(1):46–54
Pokroy R, Mimouni M, Sela T, Munzer G, Kaiserman I (2016) Myopic laser in situ keratomileusis retreatment: incidence and associations. J Cataract Refract Surg 42(10):1408–1414
Zhou J, Gu W, Li S, Wu L, Gao Y, Guo X (2020) Predictors affecting myopic regression in - 6.0D to - 10.0D myopia after laser-assisted subepithelial keratomileusis and laser in situ keratomileusis flap creation with femtosecond laser-assisted or mechanical microkeratome-assisted. Int Ophthalmol 40(1):213–225
Reinstein DZ, Ameline B, Puech M, Montefiore G, Laroche L (2005) VHF digital ultrasound three-dimensional scanning in the diagnosis of myopic regression after corneal refractive surgery. J Refract Surg 21(5):480–484
Shen Y, Chen Z, Knorz MC, Li M, Zhao J, Zhou X (2014) Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J Refract Surg 30(5):310–318
Zhu W, Han Y, Cui C, Xu W, Wang X, Dou X, Xu L, Xu Y, Mu G (2018) Corneal collagen crosslinking combined with phototherapeutic keratectomy and photorefractive keratectomy for corneal ectasia after laser in situ keratomileusis. Ophthalmic Res 59(3):135–141
Sella S, Duvdevan-Strier N, Kaiserman I (2019) Unilateral refractive surgery and myopic progression. J Pediatr Ophthalmol Strabismus 56(2):78–82
Saka N, Ohno-Matsui K, Shimada N, Sueyoshi S, Nagaoka N, Hayashi W, Hayashi K, Moriyama M, Kojima A, Yasuzumi K, Yoshida T, Tokoro T, Mochizuki M (2010) Long term changes in axial length adult eyes with pathologic myopia. Am J Ophthalmol 150(4):562–568
Gudmundsdottir E, Arnarsson A, Jonasson F (2005) Five-year refractive changes in an adult population: Reykjavik Eye Study. Ophthalmology 112(4):672–677
Fotedar R, Mitchell P, Burlutsky G, Wang JJ (2008) Relationship of 10-year change in refraction to nuclear cataract and axial length findings from an older population. Ophthalmology 115(8):1273–1278 1278e1
McBrien NA, Adams DW (1997) A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group. Refractive and biometric findings. Invest Ophthalmol Vis Sci 38(2):321–333
Fledelius HC, Goldschmidt E (2010) Oculometry findings in high myopia at adult age: considerations based on oculometric follow-up data over 28 years in a cohort-based Danish high-myopia series. Acta Ophthalmol 88(4):472–478
Pan CW, Boey PY, Cheng CY, Saw SM, Tay WT, Wang JJ, Tan AG, Mitchell P, Wong TY (2013) Myopia, axial length, and age-related cataract: the Singapore Malay eye study. Invest Ophthalmol Vis Sci 54(7):4498–4502
Chong EW, Mehta JS (2016) High myopia and cataract surgery. Curr Opin Ophthalmol 27(1):45–50
Wong YZ, Lam AK (2015) The roles of cornea and axial length in corneal hysteresis among emmetropes and high myopes: a pilot study. Curr Eye Res 40(3):282–289
Bueno-Gimeno I, España-Gregori E, Gene-Sampedro A, Lanzagorta-Aresti A, Piñero-Llorens DP (2014) Relationship among corneal biomechanics, refractive error, and axial length. Optom Vis Sci 91(5):507–513
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflicts of interest.
Ethical approval
The tenets of the Helsinki Declaration were followed in this study. It was reviewed and agreed by the Faculty of Medicine, Suez Canal University research ethics committee.
Informed consent
Informed consent was not necessary for the analysis of the medical records due to the retrospective design of the study and the large sample size.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gab-Alla, A.A. Is the axial length a risk factor for post-LASIK myopic regression?. Graefes Arch Clin Exp Ophthalmol 259, 777–786 (2021). https://doi.org/10.1007/s00417-020-04990-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04990-4