Abstract
Purpose
To investigate the predictive factors of postoperative myopic regression among subjects who have undergone laser-assisted subepithelial keratomileusis (LASEK), laser-assisted in situ keratomileusis (LASIK) flap created with a mechanical microkeratome (MM), and LASIK flap created with a femtosecond laser (FS). All recruited patients had a manifest spherical equivalence (SE) from − 6.0D to − 10.0D myopia.
Methods
This retrospective, observational case series study analyzed outcomes of refraction at 1 day, 1 week, and 1, 3, 6, and 12 months postoperatively. Predictors affecting myopic regression and other covariates were estimated with the Cox proportional hazards model for the three types of surgeries.
Results
The study enrolled 496 eyes in the LASEK group, 1054 eyes in the FS-LASIK group, and 910 eyes in the MM-LASIK group. At 12 months, from − 6.0D to − 10.0D myopia showed that the survival rates (no myopic regression) were 52.19%, 59.12%, and 58.79% in the MM-LASIK, FS-LASIK, and LASEK groups, respectively. Risk factors for myopic regression included thicker postoperative central corneal thickness (P ≦ 0.01), older age (P ≦ 0.01), aspherical ablation (P = 0.02), and larger transitional zone (TZ) (P = 0.03). Steeper corneal curvature (Kmax) (P = 0.01), thicker preoperative central corneal thickness (P < 0.01), smaller preoperative myopia (P < 0.01), longer duration of myopia (P = 0.02), with contact lens (P < 0.01), and larger optical zone (OZ) (P = 0.02) were protective factors. Among the three groups, the MM-LASIK had the highest risk of postoperative myopic regression (P < 0.01).
Conclusions
The MM-LASIK group experienced the highest myopic regression, followed by the FS-LASIK and LASEK groups. Older age, aspheric ablation used, thicker postoperative central corneal thickness, and enlarging TZ contribute to myopic regression; steeper preoperative corneal curvature (Kmax), longer duration of myopia, with contact lens, thicker preoperative central corneal thickness, lower manifest refraction SE, and enlarging OZ prevent postoperative myopic regression in myopia from − 6.0D to − 10.0D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Postoperative refractive stability has been a concern since excimer corneal refractive surgery was first introduced more than 30 years ago. The reported refraction retreatment and regression rate (RR) were different and varied from 3.8 to 66.9%, respectively [1,2,3,4,5,6]. Various regression associated factors included corneal thickness [1], age [2, 7], intraocular pressure (IOP) [8], flap thickness [9], optical zone (OZ) diameter, and thin preoperative corneal thickness of high myopia [4].
Previous studies have primarily shown the clinical outcomes of single surgery, laser-assisted in situ keratomileusis flap created with a femtosecond laser (FS-LASIK) or laser-assisted in situ keratomileusis flap created with a mechanical microkeratome (MM-LASIK) [5, 8]. Many studies have compared consequences of FS-LASIK and MM-LASIK. Some showed similar, while others favored the femtosecond laser. Most published studies applied a simple descriptive analysis, without adjustment for other factors that could affect myopic regression.
This study compared the post-surgery results for three surgery methods: LASEK, MM-LASIK, and FS-LASIK; the analysis has been done with sufficient cases for − 6.0D to − 10.0D myopia, to determine the risks and predictors of myopic regression. Multivariate analysis by stepwise regression with the Cox proportional hazard model was performed to determine predictors for myopic regression.
Methods
All data for the study were collected and analyzed in accordance with the policies of the Institutional Review Board (IRB) of the Beijing Aier-Intech Eye Hospital and in agreement with the tenets of the Declaration of Helsinki. The study protocol was approved by the ethics committee; informed consent was obtained from all individual participants in the study.
Patients
This retrospective, observational case series study enrolled myopic patients who underwent LASEK, MM-LASIK, or FS-LASIK from December 2005 to December 2013 at Beijing Aier-Intech Eye Hospital, China. A single senior surgeon performed all the surgeries. All enrolled patients had a cycloplegic spherical equivalence (SE) for − 6.0D to − 10D myopia. Inclusion criteria were over 18 years of age, had no active eye disease, a stable refractive error for at least 2 years, eyes with Schirmer test more than 5.0 mm, no history of autoimmune disease and diabetes, wanted not to use eyeglasses, and had normal corneal topography.
Data collection
The following demographic and preoperative information were extracted: a Snellen chart was used to measure uncorrected distance visual acuity (UCVA) and best-corrected distance visual acuity (BCVA), IOP by noncontact tonometer (TOPCON CT-80A; Japan), preoperative subjective sphere, preoperative subjective SE from − 6.00D to − 10.00D, preoperative subjective astigmatism up to − 6.00D by a comprehensive optometry station (TOPCON IS80, IS600; Japan); Placido-disk corneal topography, minimum K power (Kmin), maximum K power (Kmax), cylinder axis, higher-order root mean square (RMS), darkroom pupil size (OPD-Scan, ARK-10000; NIDEK, Tokyo, Japan); central corneal thickness (CCT) using a PENTECAM (TYP70700; Ocular, German). The following intraoperative information were extracted: diameter of the OZ and the transitional zone (TZ) and maximum ablation depth.
Surgical technique
All surgical procedures were performed by a single experienced surgeon (Jihong Zhou). Prior to surgery, a topical anesthetic (benoxinate hydrochloride 0.4%) was instilled twice in the conjunctival fornix of the eye. Flaps created using a 500-kHz femtosecond laser (FEMTO LDV FS; Ziemer Ophthalmic Systems AG, Port, Switzerland) had a superior hinge with an intended thickness of 110 µm and a diameter of 8.5 mm in the FS-LASIK group. Flaps created with the MK2000 (NIDEK CO., LTD., Tokyo, Japan) had a nasal hinge with a microkeratome head of 130 µm and a diameter of 8.5–9.0 mm in the MM-LASIK group. Epithelial flaps were constructed by using a self-made 8.5-mm epithelial trephine, creating epithelial dehiscence from the underlying Bowman’s membrane in the LASEK group. After exposing the corneal epithelium to 20% ethanol (in distilled water) for 12–20 s, the ethanol was absorbed with a sponge, and the cornea was thoroughly rinsed with a chilled balanced salt solution. The epithelial layer was completely dislocated by a self-made blunt blade.
After flap creation, the EC-5000 CXII 40 Hz excimer laser (NIDEK CO., LTD., Tokyo, Japan) was performed. Aspheric ablation was designed by the NIDEK NAVEK system with optimized aspheric transitional zone (OATz). All patients were fitted with soft bandage contact lenses (Acuvue Oasys, Johnson & Johnson Vision Care Inc., USA), which were removed at the end of the procedure after complete epithelization post LASEK.
Postoperative patients were prescribed fluorometholone 0.1%, ofloxacin 0.3% 4 times a day for 1 week, and artificial tears 4 times a day for 1–3 months. Patients undergoing LASEK were prescribed fluorometholone 0.1% 4 times a day for 1 month, which was tapered off and stopped at 4 months postoperatively. IOP was carefully monitored. There were no cases of corticosteroid glaucoma.
Patients were examined at 1 day, 1 week, and 1, 3, 6, and 12 months postoperatively. Refraction was recorded using an automatic refractometer (TOPCONRM-8800, Japan) at each follow-up visit. The targeted refraction was emmetropia in all eyes. Myopic regression was defined as residual myopia of − 0.50D or less and a 0.50D or more shift toward myopia during the follow-up visits. The eyes were otherwise defined as not having regression.
Statistical analysis
Data were analyzed with the IBM SPSS Statistics ver. 21 for Windows (IBM, Armonk, NY, USA). The mean value (means), standard deviation (SD), and proportions of the baseline demographic and clinical characteristics were presented. Chi square and one-way analysis of variance (ANOVA) were used to analyze the differences in the relevant clinical parameters among the three groups. A Kaplan–Meier curve was plotted to describe the postoperative refractive regression, and the log-rank test was performed to test the differences. Multivariate analysis by stepwise regression with the Cox proportional hazard model was performed to determine predictors for myopic regression.
The model can be expressed as follows:
Here \(h\left( t \right)\) is the hazard rate at time t for subject i with covariate vector (explanatory variables) Xi. The X represents the covariates (e.g., age, manifested SE, K value, etc.) and βi is the regression coefficient. The \(h_{0} \left( t \right)\) is the time-based changes at the baseline hazard function resembling the probability of myopic regression when all elucidative variables are zero. The eβi is the hazard ratio (HR) of the probability of occurrence of events in time t. In all analyses, a 2-sided P value of less than 0.05 was considered statistically significant.
Results
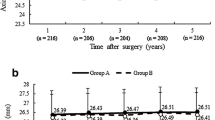
We followed up 2460 eyes: 496 in the LASEK group, 910 in the MM-LASIK group, and 1054 in the FS-LASIK group. The baseline characteristics are described in Table 1. There were no differences in the preoperative age of the three groups. The results of the univariate analysis were used to compare the demographic, preoperative, and intraoperative parameters in the LASEK, FS-LASIK, and MM-LASIK groups. Postoperative 12-month, refractive outcomes in patients who underwent LASEK, MM-LASIK, and FS-LASIK for − 6.0D to − 10.0D myopic correction are shown in Fig. 1. There was no significant intraoperative and postoperative complication in all surgeries.
Post-operative 12-month refractive outcome in patients underwent LASEK, MM-LASIK, and FS-LASIK to correct (− 6.0 to − 10.0) D myopia. LASEK = laser-assisted subepithelial keratomileusis; MM-LASIK = laser-assisted in situ keratomileusis flap creation with a mechanical microkeratome; FS-LASIK laser-assisted in situ keratomileusis flap creation with a femtosecond laser
Cumulative survival rate for − 6.0 D to − 10.0 D myopia
The cumulative survival rate of myopia regression in 12 months (no refractive regression rate) was 58.79% in the LASEK group, 59.12% in the FS-LASIK group, and 52.19% in the MM-LASIK group. The mean time of regression in the FS-LASIK group was 8.86 months, 9.71 months in the LASEK group, and 8.49 months in the MM-LASIK group for − 6.0D to − 10.0D myopia.
The univariate analysis of pre- and postoperative variables indicated that the method of surgery, age, duration of myopia, with contact lens, aspheric ablation, pre-CCT and post-CCT, preoperative corneal curvature (Kmax), preoperative manifest spherical equivalent, OZ, and TZ were significant associated factors for myopic regression. For other variables such as IOP, there were no significance with myopic regression.
The Kaplan–Meier curve showed a significant difference among the cumulative survival rates of the three groups and the results of the log-rank test (χ2 = 14.17, P < 0.001) (Fig. 2). The curve of the multivariate analyses of variables with the Cox proportional hazards model was used to further analyze the differences among the three groups (P < 0.01) (Fig. 3). Multivariate analysis with the Cox PH model gave the greater difference among three methods of surgery. The variables that were identified by the Cox proportional hazards model as significant risk factors for myopic regression were the method of surgery, age, aspheric ablation, post-CCT, and TZ. Duration of myopia, with contact lens, pre-CCT, pre-Kmax, pre-manifest SE, and OZ were ascertained as protective factors for myopic regression (Table 2).
Kaplan–Meier curves of risk for myopic regression comparing the three groups using the log-rank test in (− 6.0 to − 10.0) D myopia. LASEK = laser-assisted subepithelial keratomileusis; MM-LASIK = laser-assisted in situ keratomileusis flap creation with a mechanical microkeratome; FS-LASIK laser-assisted in situ keratomileusis flap creation with a femtosecond laser
Curves after adjusting for other covariates with the Cox proportional hazards model comparing the three groups in (− 6.0 to − 10.0) D myopia. LASEK = laser-assisted subepithelial keratomileusis; MM-LASIK = laser-assisted in situ keratomileusis flap creation with a mechanical microkeratome; FS-LASIK laser-assisted in situ keratomileusis flap creation with a femtosecond laser
Making predictions with probability of myopic regression
The predicted cumulative regression rate in each period is shown in Table 3. For patients who had MM-LASIK, the cumulative probability of myopic regression at 12 months was 59–89%. For patients who had LASEK, the cumulative probability of myopia regression at 12 months was 45–72%. For patients who had FS-LASIK, the cumulative probability of myopic regression at 12 months was 51–73%. Case 1 was an eye in a patient of 33 years old with higher myopia of − 8.00D who had MM-LASIK, spherical ablation, 5.5 mm OZ, and 7.0 mm TZ. The cumulative probability of myopic regression at 12 months was 105%. Case 2 was an eye in a patient of 31 years old with myopia of − 8.75D who had LASEK, without CL before the surgery, aspheric ablation, 5.7 mm OZ, and 7.5 mm TZ. The cumulative probability of myopic regression at 12 months was 116%. Case 3 was an eye in a patient of 32 years old with myopia of − 7.25D who had FS-LASIK, with CL before the surgery, aspheric ablation, 6.8 mm OZ, and 8.0 mm TZ. The cumulative probability of myopic regression at 12 months was 45%. On the basis of the mean covariate of all eyes in the LASEK group, FS-LASIK and MM-LASIK group, the risk was different from 45 to 89%, respectively, at 12 months.
Discussion
Since the exact etiology remains unknown, feasible mechanisms leading to myopic regression after refractive surgery have been thought to include compensatory epithelial hyperplasia [1, 10], corneal forward shift [11,12,13], nuclear sclerosis of the crystalline lens [14], and axial elongation [15,16,17].
We compared the three methods of surgery and used multivariate analysis to identify significant factors affecting myopic regression. Both risk factors and protective factors were identified. Risk factors included method of surgery, age, ablation with aspheric, post-CCT, and TZ. The duration of myopia, with contact lens, pre-CCT, preoperative manifest SE (D), and OZ were protected factors. The formula of postoperative myopic regression was predicted with the Cox proportional hazards model before the surgery by clinicians.
Methods of surgery
The manifest SE was − 8.01 ± 1.14D, − 7.55 ± 1.10D, and − 7.44 ± 1.09D, in the LASEK, FS-LASIK, and MM-LASIK groups, respectively (P < 0.001). All the different covariates including the manifest SE in the three groups were adjusted in the Cox proportional hazards model. Though greater manifest SE would present more myopic regression [18], the MM-LASIK group had the highest risk for myopic regression among the three groups, followed by FS-LASIK and LASEK groups, while the MM-LASIK group had the lowest manifest SE.
The MM-LASIK group was higher risk in myopic regression than the FS-LASIK group, which was similar to Lin et al. [18] where the flap configuration and wound adhesion between two methods might contribute to the difference. The planar flap with an FS laser had a stronger wound adhesion than the meniscus flap with a mechanical microkeratome. Kim et al. [19] found that there was more corneal stromal inflammation in the FS groups in the early postoperative period and increased flap adhesion strength later because leukocytes are a source of growth factors and cytokines, all of which are necessary for the initiation and propagation of new tissue formation in wounds [20].
Another reason that might explain our results is the cohesive tensile strength of the corneal stroma. A mathematical model based on depth-dependent stromal tensile strength data was produced by Randleman et al. [21], who found a strong negative correlation between corneal stromal depth and cohesive tensile strength. The greatest cohesive tensile strength was adjacent to Bowman’s layer; the anterior 40% of the corneal stroma had the next highest cohesive tensile strength; from 40 to 90% stromal depth, cohesive tensile strength plateaued; and from 90% stromal depth to Descemet’s membrane, cohesive tensile strength rapidly declined. Scarcelli et al. [22] and Petsche et al. [23] found a similar result for transverse shear stress, which also decreased with stromal depth.
FS-LASIK, with average flap thicknesses closer to 100 µm with less variability, may improve the safety profile of LASIK for eyes with borderline corneal thicknesses by leaving more of the anterior 40% of the corneal stroma intact [24]. MM-LASIK more frequently extends into the posterior 60% of the stroma through a combination of laser ablation and flap creation with average thicknesses of approximately 150 µm, which can be highly variable [25]. Von Jagow et al. determined that flaps in FS-LASIK were more uniform in the central and peripheral area than in MM-LASIK, and the mean thickness of the FS laser flap was significantly more accurate than the mean thickness of the microkeratome flap (P = 0.01), with a mean deviation of + 16.9 μm and 40.8 μm, respectively [26]. Cohesive tensile strength of the corneal stroma was the most affected after MM-LASIK and might be the cause of the highest regression of myopia.
The multivariate analysis showed that FS-LASIK had greater odds for regression than LASEK (HR 1.31; 95% CI 1.02–1.68; P = 0.04). Ablation beyond the anterior 40% rarely occurs with surface ablation procedures (LASEK) [25]. The LASEK procedure broke the front part of the cornea less often and kept its cohesive tensile strength better, which lead to less myopic regression.
Pre-manifest SE, OZ, TZ, and aspheric ablation
Previous studies [18, 27] produced the same result as ours that higher myopia would contribute to more myopic regression, depending on the amount of treatment.
Previous studies have shown that OZ had an important effect on myopic regression [5, 28,29,30]. Gauthier et al. [1, 31, 32] found that the epithelium with zone diameters of 6.0 mm had an epithelium thinner than 4.0–5.0 mm after photorefractive keratectomy (PRK).
Rajan et al. [30] researched PRK with 6.0-, 5.0-, and 4.0-mm OZs and indicated that a larger ablation zone gave better outcomes: less glare, halo, and regression.
The mechanism of less regression with a larger OZ might be explained by a larger ablation diameter being a smoother transition profile with a smaller peripheral step pattern, which leads to less disruption of homeostatic upper eyelid and corneal epithelial exfoliation, resulting in less epithelial hyperplasia.
We had the same result in that a larger OZ played a protective role against regression; however, a larger TZ increased the risk of regression, and spherical ablation caused less regression than aspherical ablation. Spherical ablation was designed with a large OZ and a small TZ, while aspherical ablation was done with a small OZ and a large aspheric TZ for cases of thinner corneal thickness or relatively higher myopia to save the depth of ablation.
O’Brart et al. [33] allocated 5.00-mm, 6.00-mm, or 5.00–6.00-mm multizone treatment groups with PRK. Their conclusion was that the creation of a superficial blend zone with a 5.00–6.00-mm multizone treatment had no beneficial effect on the outcome of night vision and refractive stability, but that the 6.00-mm spherical ablation diameter had a better result. Steinert et al. [34] demonstrated that there was no consistent advantage to an aspherical compared with a single-zone ablation regarding visual acuity, predictability, or stability. They designed the aspherical ablation consisting of an aspherical 5.0-mm-diameter central zone and a peripheral computer-controlled aspherical blend zone from 5.0 to 6.0 mm in diameter, with the ablation performed in a single uninterrupted pass.
Our aspherical ablation, designed by the NIDEK NAVEK system with an optimized aspheric TZ (a smaller OZ and a larger TZ, OATz), consisted of a 4.5-mm-diameter OZ and a peripheral computer-controlled (profile 1–7) aspherical TZ from 4.5 to 8.0 mm. Although a peripheral computer-controlled aspherical TZ had been optimized, stability of the postoperative refraction had not improved. Our study proved aspherical ablation (HR 1.22; 95% CI 1.03–1.44; P = 0.02) and larger TZ (HR 1.22; 95% CI 1.02–1.46; P = 0.03) generated higher odds of myopic regression. It conformed to the algorithm that a decreased OZ gave more risk of myopic regression, while an enlarged TZ was unfavorable to stability.
Pre-Kmax
We found that a higher steep pre-Kmax significantly decreased the odds for regression (HR 0.98; P = 0.01; 95% CI 0.96–0.99), similar to the findings of Chen et al.’s study [5]. Rao et al. [35] reported greater undercorrection in eyes with preoperative keratometry readings of < 43.5D than those with preoperative readings of > 44.5D. However, Russell et al. [36] found that higher K power (mean K) significantly increased the odds for regression and retreatment, because steep corneas were less stable, and therefore, they regressed more and might be corneal ectasia. However, we need to further study the safety and stability of pre-Kmax.
Duration of myopia and with contact lens
Longer duration of myopia (HR 0.98; P = 0.02; 95% CI 0.96–1.00) and with contact lens (HR 0.78; P < 0.01; 95% CI 0.67–0.91) were protective factors for postoperative myopic regression and had a negative correlation with myopic progression. Myopic progression might be caused by nuclear sclerosis of the crystalline lens [1, 14] and axial elongation [15,16,17], as noted in previous reports. Backhouse et al. [37] showed that soft spherical contact lenses create a peripheral myopic focus, unlike spectacle lenses, which create a peripheral hyperopia that could slow myopia progression.
Pre-CCT and post-CCT
We found that CCT had a significant correlation with myopic regression from 3 months to 1 year postoperatively. Before the surgery, if the CCT was thicker, there would be less myopic regression (\(\beta_{i} =\) − 0.01, P < 0.01, HR 0.99, 95% CI 0.98–0.99), similar to the findings of Lin et al. [18]. If post-CCT increased, it would give more myopic regression (\(\beta_{i}\) = 0.01, P < 0.01, HR 1.01, 95% CI 1.00–1.02), though the relationship was weak in our study. Gauthier et al. [38] showed that epithelial hyperplasia after PRK correlated with the myopic shift and contributed each D of regression to regression with 18 microns of epithelial hyperplasia. Lin et al. [39] showed a progressive myopic shift with corneal thickening 10 years after LASIK and LASEK. However, Reinstein et al. found that the epithelium continued to thicken in the central 7-mm zone by approximately 1 μm (P < 0.05) from 1 to 3 months, and no change in epithelial thickness occurred after 3 months in FS-LASIK (P > 0.05) [40].
Age
Previous reports [2, 5, 36] indicated that older-aged patients are more likely to develop regression or require retreatment, as was found in the multivariate analysis in our study. One explanation is that surgeons are careful to undercorrect with myopic presbyopia with patients who are around 40 years of age, while they tend to overcorrect for young patients [41]. The second explanation is that the lenses of older patients might develop lenticular sclerosis with a myopic shift. However, significant disagreement with this exists in Kim et al.’s [27] report that the oldest age group had a statistically significantly lower refractive regression rate than the two younger groups (35 years and < 35–45 years vs. > 45 years), because, with presbyopia, accommodative tone continues to decrease and lenticular hyperopic shifts until the age of 60 years.
The predictions of myopic regression in multivariate analysis with the Cox PH model
Figure 1 shows the absolute value of postoperative mean SE (D) (mean SE means spherical plus 1/2 cylinder). Although Fig. 1 shows a relatively stable mean SE (D), the cumulative probability (%) of myopic regression was different, which might be definition and the follow-up time after the surgery [1, 7, 18, 36, 38, 39]. In our study, the cumulative probability of myopic regression at 12 months varies from 45 to 89% in the surgeries of LASEK, FS-LASIK, and MM-LASIK, which was similar to Lin et al. [18], that myopic regression was defined as residual myopia of − 0.50D or less and a 0.50D or more shift toward myopia during 12 months. Chen et al. [5] found a myopia regression up to 6 months after MM-LASIK was 42%, lower than our study, based on the definition of regression as a − 1.0D or greater and a 0.50D or greater shift toward myopia.
In our study, the mean SE were LASEK: − 8.01 ± 1.14D, FS-LASIK: − 7.55 ± 1.10D, and MM-LASIK: − 7.44 ± 1.09D, respectively (P < 0.001), while the mean age and the use of contact lens were no statistical difference (P = 0.224, P = 0.101). Due to differences in three groups, we adjusted for all covariates, for example, duration of myopia, aspherical ablation (compare to spherical), mean manifest SE, optical zone (OZ), transition zone (TZ), etc in multivariate analysis with Cox PH model to prevent bias on heterogenous baseline. Greater manifest SE and smaller OZ would donate more myopic regression after refractive surgery though, but less approach toward myopic regression in LASEK group with the highest myopia and smallest OZ group in the three groups. It was similar to previous studies [18, 19] that the MM-LASIK was higher risk in myopic regression than the FS-LASIK and LASEK group.
Our study included three kinds of laser refractive surgery (FS-LASIK, MM-LASIK, and LASEK) while previous studies enrolled less (one or two methods of surgery) for high myopia (− 6.0D to − 10.0D) about 12 months follow-up. We included 496 eyes in the LASEK group, 1054 eyes in the FS-LASIK group, and 910 eyes in the MM-LASIK group, while the number of eyes was limited in previous studies. We used multivariate analysis with the Cox PH model and presented the cumulative probability of myopic regression in 4 periods. According to our results that predictors affect myopic regression in − 6.0D to − 10.0D after LASEK, MM-LASIK, and FS-LASIK, we could ascertain the influence of risk factors and protective factors on corneal refractive surgery. In clinical practice, we could use protective factors to stabilize postoperative diopter, ex. enlarges OZ and utilize spherical ablation; avoid risk factors to reduce postoperative regression, ex. lessens TZ; and balance the pros and cons to customize the surgical design. Importantly, we showed the HR of each covariate that we could know the impact of each factor on the risk for myopic regression. We could give patients some information when they have preoperative consultation and evaluate probability of myopic regression depending on their status in each period and the risk relevant to select a different surgery.
Limitations of our study
There are limitations to our study. First, it was inconvenient to obtain the manifested and cycloplegic refraction at each follow-up visit, as postoperative refraction was measured using an automatic optometer that was influenced by the eye accommodation. However, considering the age of our study participants (adults), the accommodation should not have markedly affected the results. Second, we had gotten the post-CCT from pre-CCT, subtracted the depth of ablation, and had not measured it, the actual post-CCT would be different. Thus, the relationship among the actual post-CCT, the calculated post-CCT, and myopic regression found in our study requires further study.
Third, this was a retrospective study; therefore, the possibility of selection bias could not be eliminated in a random clinical trial. Thus, the selection bias could not be fully avoided and some covariates are possibly confounding variables for evaluating the regression among LASEK, FS-LASIK, and MM-LASIK groups. In spite of these limitations, our results indicate that MM-LASIK was the highest risk of myopic regression in three groups in 12 months after surgery. The multivariate analyses with a Cox proportional hazards model were used and all these covariates were adjusted to minimize their influence on the prediction of LASIK regression. Predictors of myopic regression after laser refractive surgery might be assessed in a prospective study in the future.
Conclusions
For high myopia from − 6.0D to − 10.0D, the MM-LASIK group had the highest regression rate among the three groups. Using the Cox proportional hazards model, we found that the risk of postoperative myopic regression was similar in the FS-LASIK and LASEK groups. The identified risk factors for myopic regression included older age, aspherical ablation, and a larger TZ. With contact lens, lower preoperative myopia and a larger OZ were identified as protective factors, while age, duration of myopia, preoperative corneal steeper curvature (Kmax), as well as pre- and post-CCT, had a relatively weaker association. The cumulative probability provided clinicians with an accurate tool to estimate an individual’s risk for myopic regression prior to surgery.
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author upon reasonable request.
References
Chayet AS, Assil KK, Montes M, Espinosa-Lagana M, Castellanos A, Tsioulias G (1998) Regression and its mechanism after laser in situ keratomileusis in moderate and high myopia. Ophthalmology 105(7):1194–1199
Hu DJ, Feder RS, Basti S, Fung BB, Rademaker AW, Stewart P, Rosenberg MA (2004) Predictive formula for calculating the probability of LASIK enhancement. J Cataract Refract Surg 30:363–368
Albietz JM, Lenton LM, McLennan SG (2004) Chronic dry eye and regression after laser in situ keratomileusis for myopia. J Cataract Refract Surg 30:675–684
Lian J, Zhang Q, Ye W, Zhou D, Wang K (2002) An analysis of regression after laser in situ keratomileusis for treatment of myopia. Zhonghua Yan Ke Za Zhi 38(6):363–366 (in Chinese)
Chen Y-I, Chien K-L, Wang I-J, Yen AM-F, Chen L-S, Lin P-J, Chen TH-H (2007) An interval-censored model for predicting myopic regression after laser in situ keratomileusis. Invest Ophthalmol Vis Sci 48:3516–3523
Yuen LH, Chan WK, Koh J, Mehta JS, Tan DT, for the SingLasik Research Group (2010) A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in Asia. Ophthalmology 117:1236–1244.e1
Hersh PS, Fry KL, Bishop DS (2003) Incidence and associations of retreatment after LASIK. Ophthalmology 110:748–754
Qi H, Hao Y, Xia Y, Chen Y (2006) Regression-related factors before and after laser in situ keratomileusis. Ophthalmologica 220(4):272–276
Eleftheriadis H, Prandi B, Diaz-Rato A, Morcillo M, Sabater JB (2005) The effect of flap thickness on the visual and refractive outcome of myopic laser in situ keratomileusis. Eye 19:1290–1296
Lohmann CP, Reischl U, Marshall J (1999) Regression and epithelial hyperplasia after myopic photorefractive keratectomy in a human cornea. J Cataract Refract Surg 25(5):712–715
Kamiya K, Miyata K, Tokunaga T, Kiuchi T, Hiraoka T, Oshika T (2004) Structural analysis of the cornea using scanning-slit corneal topography in eyes undergoing excimer laser refractive surgery. Cornea 23(8):59–64
Baek TM, Lee KH, Kagaya F, Tomidokoro A, Amano S, Oshika T (2001) Factors affecting the forward shift of posterior corneal surface after laser in situ keratomileusis. Ophthalmology 108(2):317–320
Miyata K, Tokunaga T, Nakahara M et al (2004) Residual bed thickness and corneal forward shift after laser in situ keratomileusis. J Cataract Refract Surg 30(5):1067–1072
Fotedar R, Mitchell P, Burlutsky G, Wang JJ (2008) Relationship of 10-year change in refraction to nuclear cataract and axial length findings from an older population. Ophthalmology 115(8):1273–1278
Saka N, Ohno-Matsui K, Shimada N et al (2010) Long-term changes in axial length in adult eyes with pathologic myopia. Am J Ophthalmol 150(4):562–568
Saka N, Moriyama M, Shimada N, Nagaoka N, Fukuda K, Hayashi K, Yoshida T, Tokoro T, Ohno-Matsui K (2013) Changes of axial length measured by IOL master during 2 years in eyes of adults with pathologic myopia. Graefes Arch Clin Exp Ophthalmol 251(2):495–499
Igarashi A, Shimizu K, Kamiya K (2014) Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol 157(3):532–539
Lin MY, Chang DC, Hsu WM, Wang IJ (2012) Cox proportional hazards model of myopic regression for laser in situ keratomileusis flap creation with a femtosecond laser and with a mechanical microkeratome. J Cataract Refract Surg 38:992–999
Kim JY, Kim MJ, Kim T-I, Choi H-J, Pak JH, Tchah H (2006) A femtosecond laser creates a stronger flap than a mechanical microkeratome. Invest Ophthalmol Vis Sci 47:599–604
Baird A, Moemede P, Bohlen P (1985) Immunoreactive fibroblast growth factor in cells of peritoneal exudates suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun 126(1):358–364
Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF (2008) Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg 24(1):S85–S89
Scarcelli G, Pineda R, Yun SH (2012) Brillouin optical microscopy for corneal biomechanics. Invest Ophthalmol Vis Sci 53:185–190
Petsche SJ, Chernyak D, Martiz J, Levenston ME, Pinsky PM (2012) Depth-dependent transverse shear properties of the human corneal stroma. Invest Ophthalmol Vis Sci 53:873–880
Binder PS (2006) One thousand consecutive IntraLase laser in situ keratomileusis flaps. J Cataract Refract Surg 32:962–969
Yildirim R, Aras C, Ozdamar A, Bahcecioglu H, Ozkan S (2000) Reproducibility of corneal flap thickness in laser in situ keratomileusis using the Hansatome microkeratome. J Cataract Refract Surg 26:1729–1732
Von Jagow B, Kohnen T (2009) Corneal architecture of femtosecond laser and microkeratome flaps imaged by anterior segment optical coherence tomography. J Cataract Refract Surg 35(1):35–41
Kim G, Christiansen SM, Moshirfar M (2014) Change in keratometry after myopic laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg 40(4):564–574
O’Brart DP, Corbett MC, Lohmann CP, Kerr Muir MG, Marshall J (1995) The effects of ablation diameter on the outcome of excimer laser photorefractive keratectomy: a prospective, randomized double-blind study. Arch Ophthalmol 113:438–443
Shah SI, Hersh PS (1996) Photorefractive keratectomy for myopia with a 6-mm beam diameter. J Refract Surg 12:341–346
Rajan MS, O’Brart D, Jaycock P, Marshall J (2006) Effects of ablation diameter on long-term refractive stability and corneal transparency after photorefractive keratectomy. Ophthalmology 113(10):1798–1806
Gauthier CA, Epstein D, Holden BA, Tengroth B, Fagerholm P, Hamberg-Nyström H, Sievert R (1995) Epithelial alterations following photorefractive keratectomy for myopia. J Refract Surg 11:113–118
Gauthier CA, Holden BA, Epstein D, Tengroth B, Fagerholm P, Hamberg-Nyström H (1997) Factors affecting epithelial hyperplasia after photorefractive keratectomy. J Cataract Refract Surg 23(7):1042–1050
O’Brart DP, Corbett MC, Verma S, Heacock G, Oliver KM, Lohmann CP, Kerr Muir MG, Marshall J (1996) Effects of ablation diameter, depth, and edge contour on the outcome of photorefractive keratectomy. J Refract Surg 12(1):50–60
Steinert RF, Hersh PS (1998) Spherical and aspherical photorefractive keratectomy and laser in situ keratomileusis for moderate to high myopia: two prospective, randomized clinical trials. Summit technology PRK-LASIK study group. Trans Am Ophthalmol Soc 96:197–221 discussion 221–227
Rao SK, Cheng ACK, Fan DS, Leung AT, Lam DS (2001) Effect of preoperative keratometry on refractive outcomes after laser in situ keratomileusis. J Cataract Refract Surg 27:297–302
Pokroy R, Mimouni M, Sela T, Munzer G, Kaiserman I (2016) Myopic laser in situ keratomileusis retreatment: incidence and associations. J Cataract Refract Surg 42(10):1408–1414
Backhouse S, Fox S, Ibrahim B, Phillips JR (2012) Peripheral refraction in myopia corrected with spectacles versus contact lenses. Ophthalmic Physiol Opt 32(4):294–303
Gauthier CA, Holden BA, Epstein D, Tengroth B, Fagerholm P, Hamberg-Nyström H (1996) Role of epithelial hyperplasia in regression following photorefractive keratectomy. Br J Ophthalmol 80(6):545–548
Lim SA, Park Y, Cheong YJ, Na KS, Joo C-K (2016) Factors affecting long-term myopic regression after laser in situ keratomileusis and laser-assisted subepithelial keratectomy for moderate myopia. Korean J Ophthalmol 30(2):92–100
Reinstein DZ, Archer TJ, Gobbe M (2012) Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg 28(3):195–201
Perlman EM, Reinert SE (2004) Factors influencing the need for enhancement after laser in situ keratomileusis. J Refract Surg 20:783–789
Funding
No funding was received in relationship to this work.
Author information
Authors and Affiliations
Contributions
The contributions are as follows: study concept and design (JHZ, XHG, SWL, WG); data collection (JHZ, YG); analysis and interpretation of data (JHZ, LJW, XHG); manuscript writing (JHZ); critical revision of the manuscript (SWL, XHG); statistical expertise (JHZ, LJW, XHG); administrative, technical, or material support (JHZ, SWL, WG); and supervision (SWL, XHG, WG). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interest in the materials presented herein.
Ethics approval and consent to participate
This study was a retrospective, observational case series study that had the ethics approval and consent of the Ethics Committee of Beijing Aier-Intech Eye Hospital (No. BJAEYZ201111A01).
Consent for publication
We have these patients’ approval to publish their personal details in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhou, J., Gu, W., Li, S. et al. Predictors affecting myopic regression in − 6.0D to − 10.0D myopia after laser-assisted subepithelial keratomileusis and laser in situ keratomileusis flap creation with femtosecond laser-assisted or mechanical microkeratome-assisted. Int Ophthalmol 40, 213–225 (2020). https://doi.org/10.1007/s10792-019-01179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01179-5