Abstract
Purpose
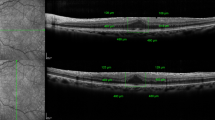
To describe functional and histopathological findings after macular peeling using different dyes.
Methods
Prospective, randomized, comparative, interventional, and immunohistochemical study. Forty-five eyes from 45 patients with idiopathic epiretinal membrane (ERM) underwent pars plana chromovitrectomy with ERM and inner limiting membrane (ILM) using trypan blue 0.15% + brilliant blue 0.05% + lutein 2% in group 1 (15 eyes), trypan blue 0.15% + brilliant blue 0.025% + polyethylene glycol 3350 4% in group 2 (15 eyes), and indocyanine green 0.05% in group 3 (15 eyes). We evaluated visual acuity (VA) and macular sensitivity (MS) preoperatively, 1, 3, and 6 months after surgery. The expression of glial fibrillary acidic protein (GFAP) and neurofilament protein (NF) was assessed immunohistochemically on the ILMs peeled as markers of glial and neuronal cells.
Results
In group 1, both mean VA and MS were significantly better at 1 and 3 months after surgery (P < 0.05), whereas no significant difference was found after 6 months. GFAP and NF expression was significantly lower in group 1 (P < 0.05).
Conclusions
The ERM/ILM peeling is thought to rip off the intraretinal tissue, based on the amounts of GFAP and NF in the specimens. The use of lutein dyes reduces iatrogenic stress to the retinal tissue and allows a faster functional recovery in the first 3 months after surgery, suggesting a less iatrogenic adhesion to the retinal tissue.

Similar content being viewed by others
References
Gupta P, Yee KM, Garcia P, Rosen RB, Parikh J, Hageman GS, Sadun AA, Sebag J (2011) Vitreoschisis in macular diseases. Br J Ophthalmol 95:376–380
Romano MR, Comune C, Ferrara M, Cennamo G, De Cillà S, Toto L, Cennamo G (2015) Retinal changes induced by epiretinal tangential forces J Ophthalmol 2015:372564
Joshi M, Agrawal S, Christoforidis JB (2013) Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediat Inflamm:192582
Kritzenberg M, Jungas B, Framme C et al (2011) Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 58:953–965
Zhao F, Gandorfer A, Haritoglou C et al (2013) Epiretinal cell proliferation in macular pucker and vitreoretinal traction syndrome: analysis of flat-mounted internal limiting membrane specimens. Retina 33:77–88
Bringmann A, Wiedemann P (2009) Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol 247:865–883
Steel DH, Lotery AJ (2013) Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 27(Suppl 1):S1–S21
Snead DRJ, James S, Snead MP (2008) Pathological changes in the vitreoretinal junction 1: epiretinal membrane formation. Eye (Lond) 22:1310–1317
Kampik A (2012) Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome 32 Suppl 2:S194–8; discussion S198–9
Romano MR, Cennamo G, Schiemer S, Rossi C, Sparnelli F, Cennamo G (2017) Deep and superficial OCT angiography changes after macular peeling: idiopathic vs diabetic epiretinal membranes. Graefes Arch Clin Exp Ophthalmol 255(4):681–689
Romano MR, Cennamo G, Cesarano I, Cardone D, Nicoletti G, Mastropasqua R, Cennamo G (2017) Changes of tangential traction after macular peeling: correlation between en face analysis and macular sensitivity. Curr Eye Res 42(5):780–788
Kenawy N, Wong D, Stappler T et al (2010) Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology 117:320–323
Junemann AG, Rejdak R, Huchzermeyer C, Maciejewski R, Grieb P, Kruse FE, Zrenner E, Rejdak K, Petzold A (2015) Elevated vitreous body glial fibrillary acidic protein in retinal diseases. Graefes Arch Clin Exp Ophthalmol 253:2181–2186
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A (2006) Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25:397–424
Lewis GP, Fisher SK (2003) Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodelling and a comparison to vimentin expression. Int Rev Cytol 230:263–290
Sousa-Martins D, Caseli L, Figueiredo MC, Sa E, Cunha C, Mota-Filipe H, Souza-Lima A, Belfort R Jr, Rodrigues E, Maia M (2015) Comparing the mode of action of intraocular lutein-based dyes with synthetic dyes. Invest Ophthalmol Vis Sci 19:1993–2000
Romano MR, Romano V, Vallejo-Garcia JL, Vinciguerra R, Romano M, Cereda M, Angi M, Valldeperas X, Costagliola C, Vinciguerra P (2014) Macular hypotrophy after internal limiting membrane removal for diabetic macular edema. Retina 34:1182–1189
Ripandelli G, Scarinci F, Piaggi P, Guidi G, Pileri M, Cupo G, Sartini MS, Parisi V, Baldanzellu S, Giusti C, Nardi M, Stirpe M, Lazzeri S (2015) Macular pucker: to peel or not to peel the internal limiting membrane? A microperimetric response. Retina 35:498–507
Petzold A, Junemana A, Reidak K et al (2009) A novel biomarker for retinal degeneration: vitreous body neurofilament proteins. J Neural Transm (Vienna) 116:1601–1606
Al-Halafi AM (2013) Chromovitrectomy: update. Saudi J Ophthalmol 27:271–276
Romano MR, Cennamo G, Amoroso F, Montorio D, Castellani C, Reibaldi M, Cennamo G (2017) Intraretinal changes in the presence of epiretinal traction. Graefes Arch Clin Exp Ophthalmol 255(1):31–38
Patel JI, Hykin PG, Schadt M, Luong V, Fitzke F, Gregor ZJ (2006) Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina 26:5–13
Eckardt C, Eckardt U, Groos S, Luciano L, Reale E (1997) Removal of the internal limiting membrane in macular holes. Clinical and morphological findings. Ophthalmologe 94:545–551
Terasaki H, Miyake Y, Nomura R, Piao CH, Hori K, Niwa T, Kondo M (2001) Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Invest Ophthalmol Vis Sci 42:229–234
Pichi F, Lembo A, Morara M, Veronese C, Alkabes M, Nucci P, Ciardella AP (2014) Early and late inner retinal changes after inner limiting membrane peeling. Int Ophthalmol 34:437–446
Romano MR, Vallejo-Garcia JL, Camesasca FI, Vincoguerra P, Costagliola C (2012) Vitreo-papillary adhesion as a prognostic factor in pseudo- and lamellar macular holes. Eye (Lond) 26:810–815
Hernandez F, Alpizar-Alvarez N, Wu L (2014) Chromovitrectomy: an update. J Ophthalmic Vis Res 9:251–259
Penha FM, Pons M, Costa EF, Rodrigues EB, Maia M, Marin-Castaño ME, Farah ME (2013) Effect of vital dyes on retinal pigmented epithelial cell viability and apoptosis: implications for chromovitrectomy. Ophthalmologica 230:41–50
Penha FM, Pons M, Costa EF Barros NM, Rodrigues EB, Cardoso EB, Dib E, Maia M, Marin-Castaño ME, Farah ME (2013) Retinal pigmented epithelial cells cytotoxicity and apoptosis through activation of the mitochondrial intrinsic pathway: role of indocyanine green, brilliant blue and implications for chromovitrectomy. PLoS One 10;8:e64094
Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, Kroemer G, Ishibashi T (2011) Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am J Pathol 179:2798–2809
Balaiya S, Brar VS, Murthy RK, Chalam KV (2011) Comparative in vitro safety analysis of dyes for chromovitrectomy: indocyanine green, brilliant blue green, bromophenol blue, and infracyanine green. Retina 31:1128–1136
Ejstrup R, la Cour M, Heegaard S, Kiilgaard JF (2012) Toxicity profiles of subretinal indocyanine green, Brilliant Blue G, and triamcinolone acetonide: a comparative study. Graefes Arch Clin Exp Ophthalmol 250:669–677
Haritoglou C, Mauell S, Benoit M, Schumann RG, Henrich PB, Wolf A, Kampik A (2013) Vital dyes increase the rigidity of the internal limiting membrane. Eye (Lond) 27:1308–1315
Wollensak G (2008) Biomechanical changes of the internal limiting membrane after indocyanine green staining. Dev Ophthalmol 42:82–90
Maia M, Furlani BA, Souza-Lima AA, Martins DS, Navarro RM, Belfort R Jr (2014) Lutein: a new dye for chromovitrectomy. Retina 34:262–272
Lüke M, Grisanti S, Lüke J; International Chromovitrectomy Collaboration (2013) The retinal biocompatibility of dyes in the ex vivo model of the isolated superfused vertebrate retina. Ophthalmologica 230 Suppl 2:21–26
Brockmann T, Steger C, Westermann M, Nietzsche S, Koenigsdoerffer E, Strobel J, Dawczynski J (2011) Ultrastructure of the membrana limitans interna after dye-assisted membrane peeling. Ophthalmologica 226:228–233
Chalam KV, Li W, Koushan K (2015) Effect of distance and duration of illumination on retinal ganglion cells exposed to varying concentrations of brilliant blue green. J Clin Med Res 7:517–524
Yanagi Y, Inoue Y, Jang WD, Kadonosono K (2006) A2e mediated phototoxic effects of endoilluminators. Br J Ophthalmol 90:229–232
Narayanan R, Kenney MC, Kamjoo S, Trinh TH, Seigel GM, Resende GP, Kuppermann BD (2005) Toxicity of indocyanine green (ICG) in combination with light on retinal pigment epithelial cells and neurosensory retinal cells. Curr Eye Res 30:471–478
Bian Q, Gao S, Jilin Z, Qin J, Taylor A, Johnson EJ, Tang G, Sparrow JR, Gierhart D, Shang F (2012) Lutein and zeaxanthin supplementation reduces hotooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic Biol Med 53:1298–1307
Sundelin SP, Nilsson SE (2001) Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radic Biol Med 31:217–225
Kijlstra A, Yuan T, Kelly ER, Berendschot TT (2012) Lutein: more than just a filter for blue light. Prog Retin Eye Res 3:303–315
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Romano, M.R., Ilardi, G., Ferrara, M. et al. Macular peeling-induced retinal damage: clinical and histopathological evaluation after using different dyes. Graefes Arch Clin Exp Ophthalmol 256, 1573–1580 (2018). https://doi.org/10.1007/s00417-018-4029-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4029-2