Abstract
Background
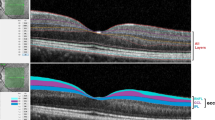
To compare peripapillary retinal nerve fiber layer thickness (RNFLT) between patients with bipolar disorder and a control group by optical coherence tomography (OCT).
Methods
This prospective comparative case series included 60 eyes of 30 patients with bipolar disorder and 60 eyes of 30 age-matched healthy control subjects. Using OCT, peripapillary RNFLT of the 4 quadrants and the mean of them was compared between the two groups. Variables such as age of onset, duration, smoking, psychosis, mania and depression episodes in the case group and their relationships with RNFLT were evaluated by OCT.
Results
Mean RNFLT was 99 ± 8 in the case group, significantly less than the106 ± 8 mμ in the control group (p = 0.001). The inferior, superior, and nasal quadrants in the case and control groups showed significant difference in RNFLT (p < 0.001) (p = 0.040) (p = 0.005); however, the temporal quadrant was not reduced significantly, compared to the control value (p = 0.907). Moreover, the only variable showing significant relation with RNFLT was duration of bipolar disorder (p = 0.040).
Conclusion
Reduction of peripapilary RNFLT occurs in patients with bipolar disorder, and is related to the duration of disease. RNFLT can be a beneficial value for studying neurodegenerative changes over time towards detecting the severity and duration of disorder.
Similar content being viewed by others
References
Begley CE, Annegers JF, Swann AC, Lewis C, Coan S, Schnapp WB et al (2001) The lifetime cost of bipolar disorder in the US. Pharmacoeconomics 19(5):483–495
Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M et al (2007) Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64(5):543–552
Sadock BJ, Sadock VA, Ruiz P (2008) Kaplan & Sadock’s concise textbook of clinical psychiatry. 9th Edition. Lippincott Williams & Wilkins, Philadelphia
Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM (2008) Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65(9):1017–1032
Vita A, De Peri L, Sacchetti E (2009) Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord 11(8):807–814
Moorhead TW, McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC et al (2007) Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry 62(8):894–900
Frey BN, Zunta-Soares GB, Caetano SC, Nicoletti MA, Hatch JP, Brambilla P et al (2008) Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? Eur Neuropsychopharmacol 18(10):717–722
Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF et al (2008) Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 63(5):465–474
Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D et al (2013) Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 170(11):1285–1296
Papiol S, Molina V, Desco M, Rosa A, Reig S, Sanz J et al (2008) Gray matter deficits in bipolar disorder are associated with genetic variability at interleukin‐1 beta gene (2q13). Genes Brain Behav 7(7):796–801
Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C et al (2008) Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry 47(5):532–539
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ (2008) Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 4(12):664–675
Bock M, Paul F, Dorr J (2013) Diagnosis and monitoring of multiple sclerosis: the value of optical coherence tomography. Nervenarzt 84(4):483–492
Frohman E, Costello F, Zivadinov R, Stuve O, Conger A, Winslow H et al (2006) Optical coherence tomography in multiple sclerosis. Lancet Neurol 5(10):853–863
Hassenstein A, Spital G, Scholz F, Henschel A, Richard G, Pauleikhoff D (2009) Optical coherence tomography for macula diagnostics. Review of methods and standardized application concentrating on diagnostic and therapy control of age-related macula degeneration. Ophthalmologe 106(2):116–126
Sergott RC, Frohman E, Glanzman R, Al-Sabbagh A (2007) The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci 263(1):3–14
Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P (2007) Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 68(18):1488–1494
Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT et al (2008) An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 131(1):277–287
De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS et al (1998) Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121(Pt 8):1469–1477
Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F (2001) Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol 112(10):1860–1867
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A (2004) Retinal nerve fiber layer thinning in Parkinson disease. Vis Res 44(24):2793–2797
Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B et al (2008) Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler 14(7):906–912
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5(2):158–170
Ascaso FJ, Cabezón L, Quintanilla MÁ, Galve LG, López-Antón R, Cristóbal JA et al (2010) Retinal nerve fiber layer thickness measured by optical coherence tomography in patients with schizophrenia: a short report. Eur J Psychiat 24(4):227–235
Cabezon L, Ascaso F, Ramiro P, Quintanilla M, Gutierrez L, Lobo A, Christobal JA (2012) Optical coherence tomography: a window into the brain of schizophrenic patients. Acta Ophthalmol 90(s249)
Lee WW, Tajunisah I, Sharmilla K, Peyman M, Subrayan V (2013) Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: evidence from optical coherence tomography. Invest Ophthalmol Vis Sci 54(12):7785–7792
Chu EM-Y, Kolappan M, Barnes TR, Joyce EM, Ron MA (2012) A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res Neuroimaging 203:89–94
APA (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publications, Washington
Sharifi Vandad ASM, Mohammadi MR, Amini H, Kaviani H, Semnani Y, Shabani A et al (2007) Structured Clinical Interview for DSM-IV (SCID): Persian translation and cultural adaptation. Iran J Psychiatry 2(1):3
Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T et al (2009) Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry 65(2):160–164
Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Hazlett EA, Haznedar MM et al (2007) A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage 37(2):449–462
Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B et al (2004) Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry 9(12):1091–1099
Bora E, Fornito A, Yücel M, Pantelis C (2010) Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry 67(11):1097–1105
Lim K, Rosenbloom M, Faustman W, Sullivan E, Pfefferbaum A (1999) Cortical gray matter deficit in patients with bipolar disorder. Schizophr Res 40(3):219–227
Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J et al (2002) Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry 159(11):1841–1847
Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A et al (2006) Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry 163(2):322–324
Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK et al (2012) Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev 36(10):2325–2333
de Azevedo-Marques PC, Duran FL, Zanetti MV, Santos LC, Murray RM, Scazufca M et al (2011) A population-based morphometric MRI study in patients with first-episode psychotic bipolar disorder: comparison with geographically matched healthy controls and major depressive disorder subjects. Bipolar Disord 13(1):28–40
Watson DR, Bai F, Barrett SL, Turkington A, Rushe TM, Mulholland CC et al (2012) Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging Behav 6(1):49–60
Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglass AW, Stoll AL (1993) Structural brain abnormalities in first-episode mania. Biol Psychiatry 33(8–9):602–609
Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW (2005) Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry 58(9):713–723
Yatham LN, Lyoo IK, Liddle P, Renshaw PF, Wan D, Lam RW et al (2007) A magnetic resonance imaging study of mood stabilizer- and neuroleptic-naive first-episode mania. Bipolar Disord 9(7):693–697
Alamouti B, Funk J (2003) Retinal thickness decreases with age: an OCT study. Br J Ophthalmol 87(7):899–901
Eriksson U, Alm A (2009) Macular thickness decreases with age in normal eyes: a study on the macular thickness map protocol in the Stratus OCT. Br J Ophthalmol 93(11):1448–1452
Yatham LN, Kapczinski F, Andreazza AC, Trevor Young L, Lam RW, Kauer-Sant’Anna M (2009) Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int J Neuropsychopharmacol 12(01):137–139
Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF et al (2012) Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res 204(1):13–24
Van Haren NE, Koolschijn PC, Cahn W, Schnack HG, Hulshoff Pol HE, Kahn RS (2010) Cigarette smoking and progressive brain volume loss in schizophrenia. Eur Neuropsychopharmacol 20(7):454–458
Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC et al (2004) Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55(1):77–84
Kuhn S, Schubert F, Gallinat J (2010) Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry 68(11):1061–1065
Conflict of interest
None of the authors has any financial/conflicting interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was carried out by Ophthalmic Research Centre Shahid Beheshti Medical University.
Ali Mehraban is a psychiatrist, Psychiatry Research Center, Department of Psychiatry, Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, and holds a MD.
Seyed Mehdi Samimi is an associate professor of Psychiatry, Psychiatry Research Center, Department of Psychiatry, Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, and holds a MD.
Morteza Entezari is an associate professor of Ophthalmology, Ophthalmic Research Center, Department of Ophthalmology, Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, and holds a MD.
Mohammad Hassan Seifi is an ophthalmologist, Ophthalmic Research Center, Department of Ophthalmology, Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, and holds a MD.
Maryam Nazary is a neurologist, Department of Neurology, Imam Hossein Medical Center, Shahid Beheshti University of Medical Sciences, and holds a MD.
Mehdi Yaseri is a biostatistician, Department of Epidemiology and Biostatistics, Tehran University of Medical Sciences, and holds a PhD.
Rights and permissions
About this article
Cite this article
Mehraban, A., Samimi, S.M., Entezari, M. et al. Peripapillary retinal nerve fiber layer thickness in bipolar disorder. Graefes Arch Clin Exp Ophthalmol 254, 365–371 (2016). https://doi.org/10.1007/s00417-015-2981-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2981-7